Electron Shells, Subshells, and Orbitals
CONTENT INDEX
Electron Shells History
- The shell terminology comes from Arnold Sommerfeld’s modification of the Bohr model.
- Sommerfeld retained Bohr’s planetary version, however added mildly elliptical orbits (characterized with the aid of way of extra quantum numbers ℓ and m) to supply a rationalization for the brilliant spectroscopic shape of some factors.
- A couple of electrons with the equal important quantum range (n) had close to orbits that usual a “shell” of top-notch thickness as hostile to the infinitely skinny spherical orbit of Bohr’s version.
- The existence of electron shells used to be first found experimentally in Charles Barkla’s and henry Moseley’s x-ray absorption research.
- Barkla classified them with the letters k, l, m, n, o, p, and q.
- The starting place of this terminology grew to be alphabetic.
- A “j” series used to be in addition suspected, even although later experiments indicated that the k absorption traces are produced through the innermost electrons. Those letters had been later found to correspond to the n values 1, 2, 3, and many others. They are used in the spectroscopic Siegbahn notation.
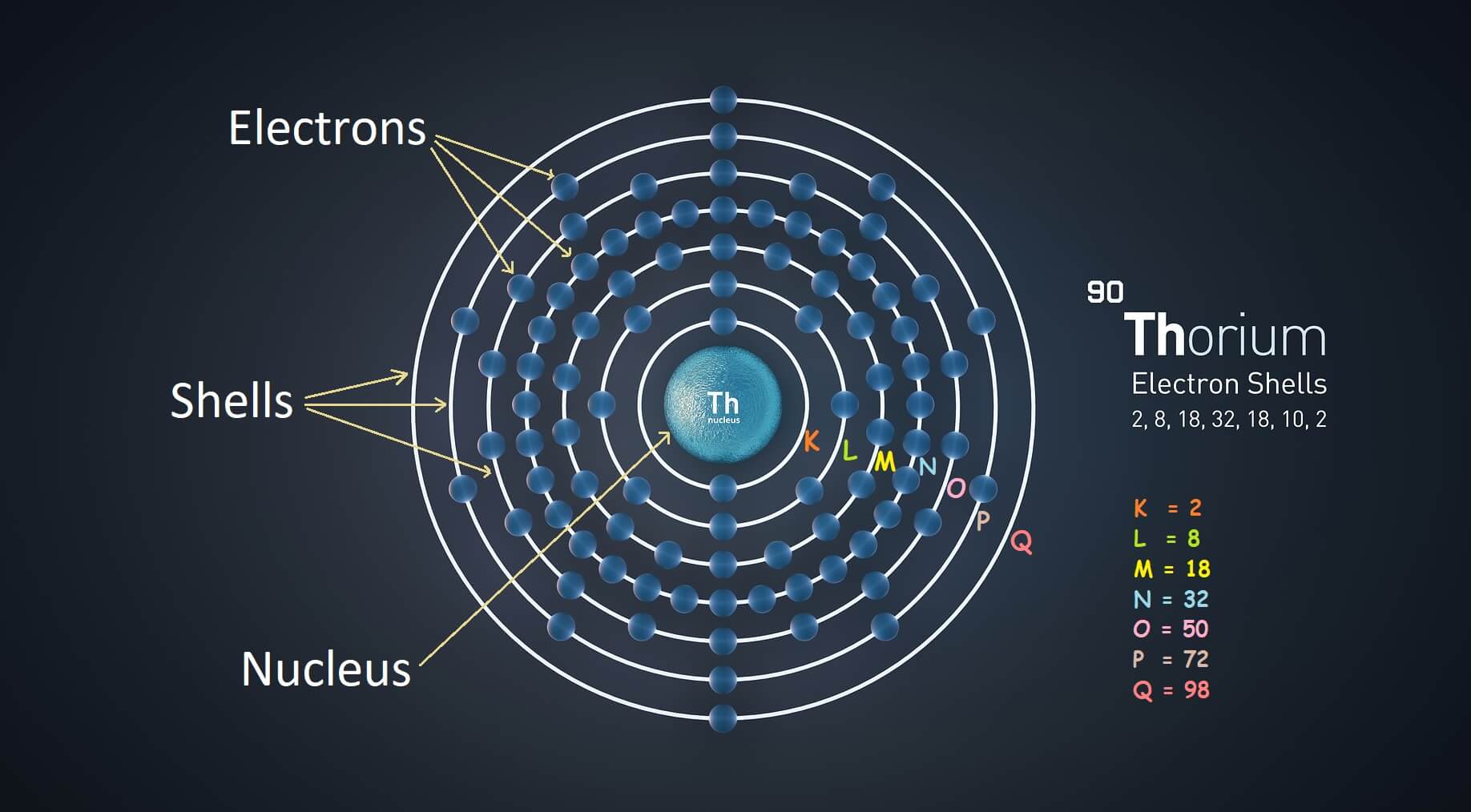
Electron Shells
According to Bohr’s atomic model, there are fixed paths around the nucleus where electrons revolve. He called these paths are shells. There are four types of electron shells which are following:
- First shell of electron is known as the K shell.
- Second shell is known as the L shell.
- Third shell is known as the M shell.
- Fourth shell is known as the N shell.
To sum up this whole concept we can write as:
- When n=1, It is first shell which is known as the K shell. K shell has maximum 2 electrons.
- When n=2, It means that, it is second shell which is L shell. It has maximum 8 electrons.
- When n=3, It is third shell are M shell. It is consisting of maximum 18 electrons.
- When n=4, it is N shell which are fourth shell. It has maximum 32 electrons.
- When n=5, it is O shell which are fifth shell. It has maximum 50 electrons.
- When n=6, it is P shell which are sixth shell. It has maximum 72 electrons.
- When n=7, it is Q shell which are seventh shell. It has maximum 98 electrons.
Every shell is divided into sub shell, Where K, L, M, N are main shells, while s, p, d, f are sub shells.
Maximum number of electron calculation:
Formula: Maximum number of electrons in a shell= 2n2.
Electron Sub-shells
A sub-orbit is a sub division of electron shell (orbit) separated by electron orbitals.
- Sub-orbit also known as sub shells or sub energy level.
- Subshells are denoted by s, p, d, f.
- This subshells s, p, d, f is known as sharp, principle, diffuse, fundamental respectively.
- There are four subshells, where each subshell has different numbers of electrons.
| Subshell | Electrons |
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
- The n number determines that how many of the subshells make up the shell. For example: the 1st shell is consisting of one subshell s. It can consequently include only two electrons.
- The 2nd shell is made up of two subshells, s and p. It can consequently comprise 2+6=8 electrons.
| Shell | Subshell | Total Number of Electrons in Shell |
| 1st Shell (K shell) | 1s | 2 |
| 2nd Shell (L shell) | 2s, 2p | 2 + 6 = 8 |
| 3rd Shell (M shell) | 3s, 3p, 3d | 2 + 6 + 10 = 18 |
| 4th Shell (N shell) | 4s, 4p, 4d, 4f | 2 + 6 + 10 + 14 = 32 |
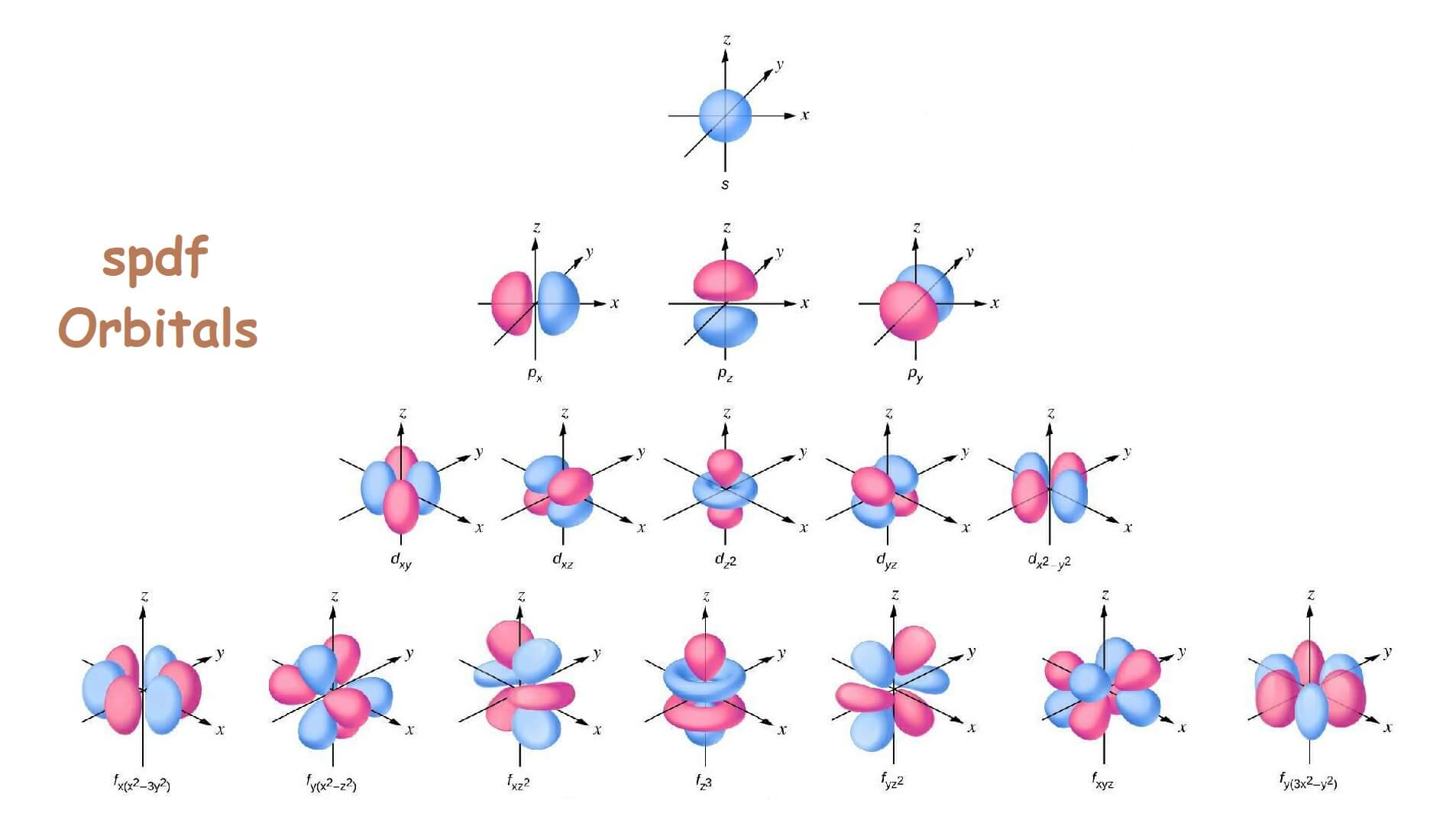
What are orbitals?
It is a three-dimensional probability space around the nucleus of an atom where the possibility of finding the electron cloud is maximum (95%).
Types of orbitals
1. s orbital: It is spherical in shape. It has only one orbital.
2. p orbital: p sub-shell consists of three orbitals which are px, py and pz.
3. d orbital: d subshell consists of five orbitals (dxy, dyz, dzx, dz2, dx2– y2)
4. f orbital: f subshell consists of seven orbitals (fx(x2– 3y2), fy(x2– z2), fxz2, fz3, fyz2, fxyz, fy(3x2– y2)). They shows different directions in magnetic field.
List of Elements with Electrons Per Shell
| Atomic number[z] | Element | No. of electrons Per shell | Group |
| 1 | Hydrogen | 1 | 1 |
| 2 | Helium | 2 | 18 |
| 3 | Lithium | 2, 1 | 1 |
| 4 | Beryllium | 2, 2 | 2 |
| 5 | Boron | 2, 3 | 13 |
| 6 | Carbon | 2, 4 | 14 |
| 7 | Nitrogen | 2, 5 | 15 |
| 8 | Oxygen | 2, 6 | 16 |
| 9 | Fluorine | 2, 7 | 17 |
| 10 | Neon | 2, 8 | 18 |
| 11 | Sodium | 2, 8, 1 | 1 |
| 12 | Magnesium | 2, 8, 2 | 2 |
| 13 | Aluminium | 2, 8, 3 | 13 |
| 14 | Silicon | 2, 8, 4 | 14 |
| 15 | Phosphorus | 2, 8, 5 | 15 |
| 16 | Sulfur | 2, 8, 6 | 16 |
| 17 | Chlorine | 2, 8, 7 | 17 |
| 18 | Argon | 2, 8, 8 | 18 |
| 19 | Potassium | 2, 8, 8, 1 | 1 |
| 20 | Calcium | 2, 8, 8, 2 | 2 |
| 21 | Scandium | 2, 8, 9, 2 | 3 |
| 22 | Titanium | 2, 8, 10, 2 | 4 |
| 23 | Vanadium | 2, 8, 11, 2 | 5 |
| 24 | Chromium | 2, 8, 13, 1 | 6 |
| 25 | Manganese | 2, 8, 13, 2 | 7 |
| 26 | Iron | 2, 8, 14, 2 | 8 |
| 27 | Cobalt | 2, 8, 15, 2 | 9 |
| 28 | Nickel | 2, 8, 16, 2 | 10 |
| 29 | Copper | 2, 8, 18, 1 | 11 |
| 30 | Zinc | 2, 8, 18, 2 | 12 |
| 31 | Gallium | 2, 8, 18, 3 | 13 |
| 32 | Germanium | 2, 8, 18, 4 | 14 |
| 33 | Arsenic | 2, 8, 18, 5 | 15 |
| 34 | Selenium | 2, 8, 18, 6 | 16 |
| 35 | Bromine | 2, 8, 18, 7 | 17 |
| 36 | Krypton | 2, 8, 18, 8 | 18 |
| 37 | Rubidium | 2, 8, 18, 8, 1 | 1 |
| 38 | Strontium | 2, 8, 18, 8, 2 | 2 |
| 39 | Yttrium | 2, 8, 18, 9, 2 | 3 |
| 40 | Zirconium | 2, 8, 18, 10, 2 | 4 |
| 41 | Niobium | 2, 8, 18, 12, 1 | 5 |
| 42 | Molybdenum | 2, 8, 18, 13, 1 | 6 |
| 43 | Technetium | 2, 8, 18, 13, 2 | 7 |
| 44 | Ruthenium | 2, 8, 18, 15, 1 | 8 |
| 45 | Rhodium | 2, 8, 18, 16, 1 | 9 |
| 46 | Palladium | 2, 8, 18, 18 | 10 |
| 47 | Silver | 2, 8, 18, 18, 1 | 11 |
| 48 | Cadmium | 2, 8, 18, 18, 2 | 12 |
| 49 | Indium | 2, 8, 18, 18, 3 | 13 |
| 50 | Tin | 2, 8, 18, 18, 4 | 14 |
| 51 | Antimony | 2, 8, 18, 18, 5 | 15 |
| 52 | Tellurium | 2, 8, 18, 18, 6 | 16 |
| 53 | Iodine | 2, 8, 18, 18, 7 | 17 |
| 54 | Xenon | 2, 8, 18, 18, 8 | 18 |
| 55 | Caesium | 2, 8, 18, 18, 8, 1 | 1 |
| 56 | Barium | 2, 8, 18, 18, 8, 2 | 2 |
| 57 | Lanthanum | 2, 8, 18, 18, 9, 2 | |
| 58 | Cerium | 2, 8, 18, 19, 9, 2 | |
| 59 | Praseodymium | 2, 8, 18, 21, 8, 2 | |
| 60 | Neodymium | 2, 8, 18, 22, 8, 2 | |
| 61 | Promethium | 2, 8, 18, 23, 8, 2 | |
| 62 | Samarium | 2, 8, 18, 24, 8, 2 | |
| 63 | Europium | 2, 8, 18, 25, 8, 2 | |
| 64 | Gadolinium | 2, 8, 18, 25, 9, 2 | |
| 65 | Terbium | 2, 8, 18, 27, 8, 2 | |
| 66 | Dysprosium | 2, 8, 18, 28, 8, 2 | |
| 67 | Holmium | 2, 8, 18, 29, 8, 2 | |
| 68 | Erbium | 2, 8, 18, 30, 8, 2 | |
| 69 | Thulium | 2, 8, 18, 31, 8, 2 | |
| 70 | Ytterbium | 2, 8, 18, 32, 8, 2 | |
| 71 | Lutetium | 2, 8, 18, 32, 9, 2 | 3 |
| 72 | Hafnium | 2, 8, 18, 32, 10, 2 | 4 |
| 73 | Tantalum | 2, 8, 18, 32, 11, 2 | 5 |
| 74 | Tungsten | 2, 8, 18, 32, 12, 2 | 6 |
| 75 | Rhenium | 2, 8, 18, 32, 13, 2 | 7 |
| 76 | Osmium | 2, 8, 18, 32, 14, 2 | 8 |
| 77 | Iridium | 2, 8, 18, 32, 15, 2 | 9 |
| 78 | Platinum | 2, 8, 18, 32, 17, 1 | 10 |
| 79 | Gold | 2, 8, 18, 32, 18, 1 | 11 |
| 80 | Mercury | 2, 8, 18, 32, 18, 2 | 12 |
| 81 | Thallium | 2, 8, 18, 32, 18, 3 | 13 |
| 82 | Lead | 2, 8, 18, 32, 18, 4 | 14 |
| 83 | Bismuth | 2, 8, 18, 32, 18, 5 | 15 |
| 84 | Polonium | 2, 8, 18, 32, 18, 6 | 16 |
| 85 | Astatine | 2, 8, 18, 32, 18, 7 | 17 |
| 86 | Radon | 2, 8, 18, 32, 18, 8 | 18 |
| 87 | Francium | 2, 8, 18, 32, 18, 8, 1 | 1 |
| 88 | Radium | 2, 8, 18, 32, 18, 8, 2 | 2 |
| 89 | Actinium | 2, 8, 18, 32, 18, 9, 2 | |
| 90 | Thorium | 2, 8, 18, 32, 18, 10, 2 | |
| 91 | Protactinium | 2, 8, 18, 32, 20, 9, 2 | |
| 92 | Uranium | 2, 8, 18, 32, 21, 9, 2 | |
| 93 | Neptunium | 2, 8, 18, 32, 22, 9, 2 | |
| 94 | Plutonium | 2, 8, 18, 32, 24, 8, 2 | |
| 95 | Americium | 2, 8, 18, 32, 25, 8, 2 | |
| 96 | Curium | 2, 8, 18, 32, 25, 9, 2 | |
| 97 | Berkelium | 2, 8, 18, 32, 27, 8, 2 | |
| 98 | Californium | 2, 8, 18, 32, 28, 8, 2 | |
| 99 | Einsteinium | 2, 8, 18, 32, 29, 8, 2 | |
| 100 | Fermium | 2, 8, 18, 32, 30, 8, 2 | |
| 101 | Mendelevium | 2, 8, 18, 32, 31, 8, 2 | |
| 102 | Nobelium | 2, 8, 18, 32, 32, 8, 2 | |
| 103 | Lawrencium | 2, 8, 18, 32, 32, 8, 3 | 3 |
| 104 | Rutherfordium | 2, 8, 18, 32, 32, 10, 2 | 4 |
| 105 | Dubnium | 2, 8, 18, 32, 32, 11, 2 | 5 |
| 106 | Seaborgium | 2, 8, 18, 32, 32, 12, 2 | 6 |
| 107 | Bohrium | 2, 8, 18, 32, 32, 13, 2 | 7 |
| 108 | Hassium | 2, 8, 18, 32, 32, 14, 2 | 8 |
| 109 | Meitnerium | 2, 8, 18, 32, 32, 15, 2 | 9 |
| 110 | Darmstadtium | 2, 8, 18, 32, 32, 16, 2 | 10 |
| 111 | Roentgenium | 2, 8, 18, 32, 32, 17, 2 | 11 |
| 112 | Copernicium | 2, 8, 18, 32, 32, 18, 2 | 12 |
| 113 | Nihonium | 2, 8, 18, 32, 32, 18, 3 | 13 |
| 114 | Flerovium | 2, 8, 18, 32, 32, 18, 4 | 14 |
| 115 | Moscovium | 2, 8, 18, 32, 32, 18, 5 | 15 |
| 116 | Livermorium | 2, 8, 18, 32, 32, 18, 6 | 16 |
| 117 | Tennessine | 2, 8, 18, 32, 32, 18, 7 | 17 |
| 118 | Oganesson | 2, 8, 18, 32, 32, 18, 8 | 18 |
that’s all summary about electron shells.