33 As (Arsenic)

Arsenic is a steel gray, crystalline, very brittle, semimetallic solid element.
It appears in three allotropic forms: yellow, black and grey.
It tarnishes in air, and when it is heated it rapidly oxidizes to arsenic trioxide (As2O3), which smells like of garlic.
The non metallic form of arsenic is less reactive but it dissolve when heated with strong oxidizing acids and alkalis.



Identity
CAS Number: CAS7440-38-2
CID Number: CID5359596
DOT Hazard Class: 6.1
DOT Number: 1558
RTECSCG0525000
CONTENT INDEX
Basic Properties of Arsenic
Pronunciation: Ars-nik / Ar-sen-ik
Appearance: Metallic grey
Allotropes: Grey, yellow, black Arsenic
Mass Number: 75
Standard Atomic weight: 74.921 g/mol
Atomic number (Z): 33
Electrons: 33
Protons: 33
Neutrons: 42
Period: 4
Group: 15
Block: p
Element category: Metalloids
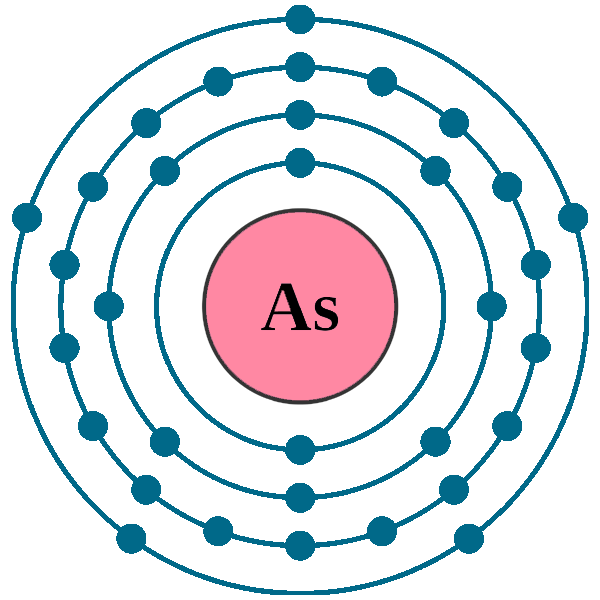
Electrons per shell: K2, L8, M18, N5
Electron configuration: 1s22s22p63s23p63d104s24p3
Thermal Properties of Arsenic
Phase: Solid
Melting point: 1315 K (1042 oC, 1908 oF)
Boiling point: 3273 K (1900 oC, 5432 oF)
Debye temperature: 285 K (11.85 oC, 53.33 oF)
Sublimation point: 887 K (615 oC, 1137 oF)
Triple point temperature: 1090 K (816.85 oC, 1502.33 oC)
Triple point pressure: 3628 kPa (35.805 Atm)
Critical point temperature: 1673 K (1399.85 oC, 2551.73 oF)
Fusion heat: grey: 24.44 kJ/mol
Vaporization heat: 34.76 kJ/mol
Specific heat: 328 J/(kg K)
Molar heat capacity: 24.64 J/(mol.K)
Thermal expansion: 5.6 μm/(m∙K)
Thermal conductivity: 50.2 W/(m∙K)
Electrical properties of Arsenic
Electrical conductivity: 3.3×106 S/m
A Electrical resistivity: 333 μΩ∙m
A Electrical type: Conductor
Magnetic Properties of Arsenic
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -5.5×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000223
Mass magnetic susceptibility: -3.9×10-9 m3/kg
Molar magnetic susceptibility: -0.292×10-9 m3/mol
Physical Properties of Arsenic
Density: 5.727 g/cm3 (In solid) 5.22 g/cm3 (In Liquid)
Molar volume: 0.00002045 m3/mol
Refractive Index: 1.001552 (Ratio of the velocity of light in a vacuum to its velocity in a specified medium)
Young’s modulus: 8 GPa
Mohs Hardness: 3.5
Bulk modulus: 22 GPa
Vicker hardness: 1510 MPa
Brinell hardness: 1440 MPa
Atomic Properties of Arsenic
Oxidation states: 5,4, 3, 2, 1, -1, -2, -3
Valence Electrons: 4s2 4p3
Ion charge: As3-
The ionization potential of an atom: 9.96
Ionization energies: 1st: 947 kJ.mol 2nd: 1798 kJ/mol 3rd: 2735 kJ/mol
Ionic radius: 58 pm
Atomic radius: empirical: 119 pm
Van der Waals: 185 Pm
Covalent radius: 119±4 pm
Filling Orbital: 4p3
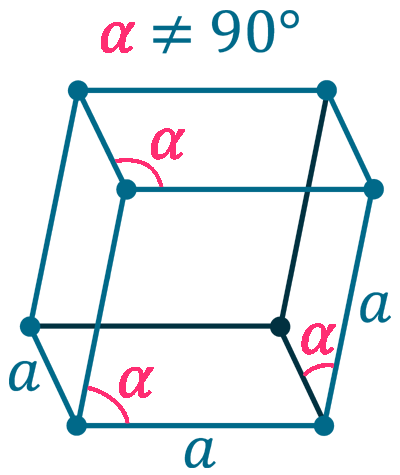
Crystal structure: Rhombohedral
Grid parameters: a=0.4123 HM. α=54.17
Space Group Name: R_3m
Space Group Number: 166
Reactivity of Arsenic
Electronegativity: pauling scale: 2.18
Valence: +5
Electron affinity: 78 kJ/mol
Nuclear Properties of Arsenic
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 4S3/2
Neutron cross section (Brans): 4.2
Neutron Mass Absorption: 0.002
Isotopes: 73As 74As 75As
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 73As | Syn | – | 80.3 d |
| 74As | Syn | – | 17.8 d |
| 75As | 100 | 74.922 | Stable |
Chemical Reactions of Arsenic
AArsenic does not react with dry air at room temperature, but the surface oxidizes slowly when react with moist air, and forming arsenice (V) oxide. When heated in air, arsenic ignites and also forming arsenic (V) oxide:
4 As (s) 5 O2 (g) → 2 As2O5 (s)
When heat in a pure oxygen atmosphere, forming arsenic (III) oxide:
4 As (s) + 3 O2 (g) → As4O6 (s)
AArsenic does not react with water in the absence of air under normal conditions:
Reacts with fluorine, and forming Arsenic (V) fluoride:
2 As (s) + 5 F2 (g) → 2 AsF5 (g) [colourless]
Reacts with all Halogens under controlled conditions:
2 As (s) + 3 F2 (g) → 2 AsF3 (l) [colourless] (Arsenic (lll) fluoride)
2 As (s) + 3 Cl2 (g) → 2 AsCl3 (l) [colourless] (Arsenic (lll) chloride)
2 As (s) + 3 Br2 (g) → 2 AsBr3 (s) [pale yellow] (Arsenic (lll) bromide)
2 As (s) + 3 I2 (g) → 2 AsI3 (s) [red] (Arsenic (lll) iodide)
Arsenic History
Naming: Greek: arsenikos (male); Latin: arsenicum.
Discovery: Before 300 CE
Discoverer: Albertus Magnus (in 1250)
Arsenic Uses
AArsenic is used in bronzing (bronze-like surface is applied to other materials), pyrotechny (making fireworks), and for hardening and improving the sphericity of shot.
It is used in making special types of glass, as wood preservative, and as a doping agent in solid-state devices such as transistors.
Semiconductors (gallium arsenide, GaAs) is used as a laser material to convert electricity directly into coherent light.
Some arsenic compounds are used as agricultural insecticides and poisons.
The most important compounds are white arsenic (As2O3), the sulfide (As2S3) , Paris green (copper acetoarsenite, Highly toxic, Cu(C2H3O2)2·3Cu(AsO2)2) , calcium arsenate (Ca3(AsO4)2), and lead arsenate (inorganic insecticide, PbHAsO4).
Some aarsenic compounds have been used as medicines.
In 18th , 19th century, Dr Fowler’s Solution (potassium arsenate (KAsO2) dissolved in water) was a popular cure-all tonic that was even used by Charles Dickens.
Today, organoarsenic compounds are added in poultry feed to prevent disease and improve weight gain.
Marsh test is a highly sensitive method in the detection of aarsenic, It makes use of the formation and ready decomposition of arsine.
Biological role of Arsenic
AArsenic and its compounds are poisonous, even in small amount of doses it is a suspected carcinogen (a substance capable of causing cancer in living tissue).
If it is inside the body, it bonds to atoms in the hair, so by analysing hair samples can show whether someone has been exposed to arsenic.
Humans may be exposed to aarsenic through food, water and air, also through skin contact with soil or water that contains arsenics.
Fish absorb arsenic from the water they live in, thatswhy levels of arsenic in fish and seafood (prawns) may be high.
Luckly it has the fairly harmless organic form of arsenic, but if the fish contain significant amounts of inorganic arsenic, It may be danger to human health.
Abundance of Arsenic
AArsenic can be found naturally on earth in small concentrations.
It occurs in soil (100 mg/kg) and minerals and it may enter air (not exceed 3 ng/m3), water (10 μg/L) and land through wind-blown dust and water run-off.
The most common aarsenic-containing mineral is arsenopyrite (Iron arsenic sulfide, FeAsS), Others are include realgar (As4S4 or AsS), orpiment (As2S3), and enargite (Cu3AsS4).
On heating arsenopyrite in air, aArsenic sublimes (transition of a substance directly from the solid to the gas phase) as arsenic (III) oxide and leaving iron (II) sulfide, and if heated it without air, gray arsenic will produce.
microorganisms release volatile methylarsines to the extent of 20.000 tonnes per year.
vulcanoes release about 3000 tonnes per year.
80.000 tonnes of arsenic per year are released by the burning of fossil fuels.
Annual world wide production is around 50,000 tons in form of Compound (Arsenic tri-oxide, As2O3)
8×10-7% (In Universe)
18×10-5% (In Meteorites)
0.00021% (In Earth’s Crust)
2.3×10-7% (In Oceans)
5×10-6% (In Humans)




World’s Top 3 producers of Arsenic
1) China
2) Morocco
3) Russia
#Arsenic


