15 P (Phosphorus Element)

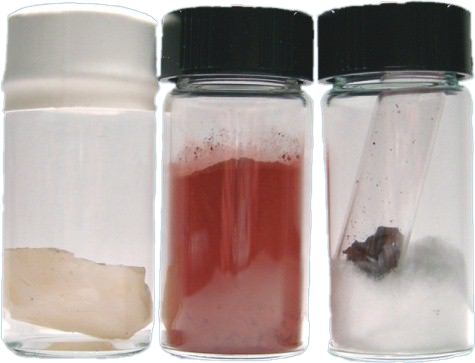
Phosphorous is a multivalent nonmetal of the nitrogen group, and it is found in nature in four or more allotropic forms (white or yellow, red or orange, black or violet phosphorus), where Ordinary phosphorus is a waxy white solid and the pure form of phosphorus is colorless or transparent.
It is an essential element for the life of organisms.
White phosphorus has two forms, alpha (α) and beta (β) with a transition temperature at (-3.8°C).
It is insoluble in water (H2O), but soluble in carbon disulfide (CS2).
It glows in the dark and takes fire spontaneously in air, burning to the pentoxide (P4O10).
Black phosphorous, is made under high pressure and it looks like graphite and has the ability to conduct electricity like graphite.
Identity
CAS Number: CAS7723-14-0
CID Number: CID5462309
DOT Hazard Class: 4.1
DOT Number: 1338, 1381, 2447
RTECS Number: RTECSTH3495000
CONTENT INDEX
Basic Properties of Phosphorus
Allotropes: White phosphorus, Red phosphorus, Black phosphorus
Pronunciation: Fos-far-as
Appearance: colourless, waxy white, scarlet, yellow, violet, red, black
Mass Number: 31
Standard Atomic weight: 30.974 g/mol
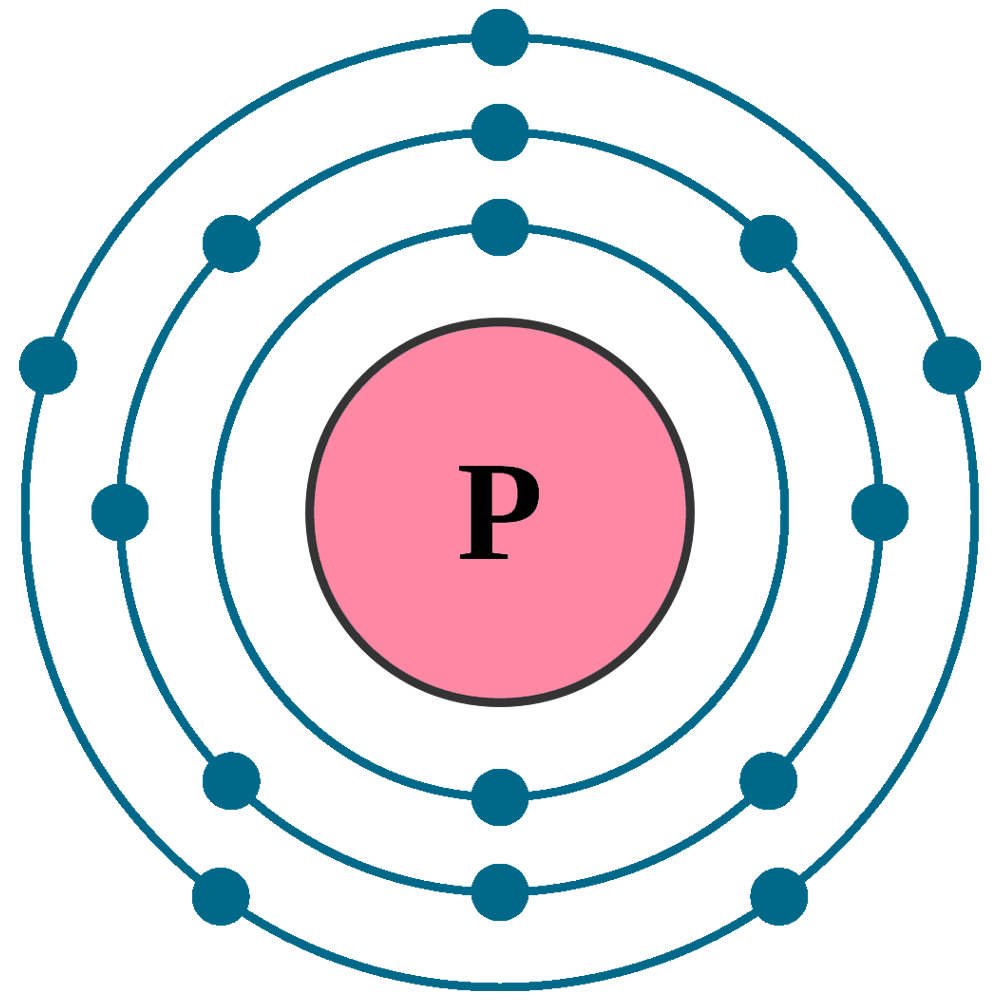
Atomic number (Z): 15
Electrons: 15
Protons: 15
Neutrons: 16
Period: 3
Group: 15
Block: p
Element category: Reactive Non-metal
Electrons per shell: K2, L8, M5
Electron configuration: 1s22s22p63s23p3
Thermal Properties of Phosphorus
Phase: Solid
Melting point: white: 317.3 K (44.15 oC, 111.5 oF) Red: ~860 K (~590 oC, ~1090 oF)
Boiling point: White: 553.7 K (280.5 oC, 536.9 oF)
Fusion heat: white: 0.66 kJ/mol
Vaporization heat: white: 51.9 kJ/mol
Specific heat: 769.6 J/(kg K)
Molar heat capacity: white: 23.824 J/(mol.K)
Thermal conductivity: white: 0.236 W/(m∙K), black: 12.2 W/(m∙K)
Electrical properties of Phosphorus
Electrical conductivity: 10×106 S/m
A Electrical resistivity: 1×10-7 Ω∙m
A Electrical type: Conductor
Magnetic Properties of Phosphorus
A Magnetic type: Diamagnetic (white, violet, red, black)
Curie point: (above which Ferromagnetism vanishes)
Magnetic susceptibility (xmol): -20.8×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000206
Mass magnetic susceptibility: -0.113.x10-9 m3/kg
Molar magnetic susceptibility: -35×10-9 m3/mol
Physical Properties of Phosphorus
Density: white: 1.823 g/cm3, violet: 2.36 g/cm3, red: ~2.2-2.34 g/cm3, black: 2.69 g/cm3
Molar volume: 0.000016991 m3/mol
Bulk modulus: white: 5 GPa, red: 11 GPa
Refractive Index: 1.001212
Sound Speed: 2130 m/s
Atomic Properties of Phosphorus
Oxidation states: –3, -2, -1, 1, 2, 3, 4, 5
Valence Electrons: 3s2 3p3
Ion charge: P3-
Ionization potential of an atom: 10.3
Ionization energies: 1st: 1012 kJ.mol 2nd: 1907 kJ/mol 3rd: 2914 kJ/mol
Ionic radius: 38 pm
Atomic radius: 98 pm (empirical)
Van der Waals: 180 Pm
Covalent radius: 107±3 pm
Filling Orbital: 3p3
Crystal structure: body centred cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 1880, 1880, 1880 pm
Grid parameters: a=18.800 Å
Space Group Name: P_1
Space Group Number: 2

Reactivity of Phosphorus
Electronegativity: 2.19 (pauling scale)
Valence: +5
Electron affinity: 71 kJ/mol
Nuclear Properties of Phosphorus
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 4S3/2
Neutron cross section (Brans): 0.0002
Neutron Mass Absorption: 4.7
Isotopes: 31P 32P 33P
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 31P | 100 | 30.974 | Stable |
| 32P | Trace | – | 14.28 d |
| 33P | Trace | – | 25.3 d |
Chemical Reactions of Phosphorus
APhosphorus ignites spontanteously in air at room temperature, and forming phosphorus pentoxide (actually tetraphosphorous decaoxide, P4O10):
P4 (s) + 5 O2 (g) → P4 O10 (s)
Under controlled conditions (25% N2, 75% O2, 50o C, 90 mm Hg), a mixture is formed, which containing phosphorous trioxide (actually tetraphosphorous hexaoxide, P4O6)
P4(s) + 3 O2 (g) → P4O6 (s)
It doesn’t react with water and dilute non-oxidizing acids:
Phosphorus reacts with excess Fluorine (F2), and forming phosphorus (V) fluoride:
2 P (s) + 5 F2 (g) → 2 PF5 (s)
Reacts with Fluorine with excess phosphorus, It forms:
2 P (s) + 3 F2 (g) → 2 PF3 (s)
PF3 (s) + F2 (g) → PF5 (s)
Phosphorus reacts with excess Chlorine (Cl2), and forming phosphorus (V) chloride:
2 P (s) + 5 Cl2 (g) → 2 PCl5 (s)
And reacts with Chlorine with excess phosphorus:
2 P (s) + 3 Cl2(g) → 2 PCl3 (s) (phosphorus trichloride)
PCl3 (s) + Cl2 (g) → PCl5 (s) (phosphorus pentachloride)
Phosphorus reacts with excess Bromine (Br2) , and forming phosphorus (V) bromide:
2 P (s) + 5 Br2 (l) → 2 PBr5 (s)
Reacts with Bromine with excess phosphorus:
2 P (s) + 3 Br2 (l) → 2 PBr3 (s)
PBr3 (s) + Br2 (l) → PBr5 (s)
Phosphorus doesn’t react with Iodine (I2):
Production
Phosphate rock (calcium phosphate) can be heated to 1200-1500 o C with sand (SiO2) and coke (refined coal) to produced P4 (vaporised), and after it condensed into a white powder under water (prevent oxidation in air):
4 Ca5(PO4)3F + 18 SiO2 + 30 C → 3 P4 + 30 CO + 18 CaSiO3 + 2 CaF2
Reaction of Another process by which phosphorus is extracted by applying high temperature (1500o C):
2 Ca3(PO4)2 + 6 SiO2 + 10 C → 6 CaSiO3 + 10 CO + P4
In this reaction, Tricalcium phosphate (Ca3(PO4)2) in bone ash is converted to monocalcium phosphate (Ca(H2PO4)2) with sulfuric acid (H2SO4):
Ca3(PO4)2 + 2 H2SO4 → Ca(H2PO4)2 + 2 CaSO4
In this reaction Monocalcium phosphate is dehydrated to the corresponding metaphosphate:
Ca(H2PO4)2 → Ca(PO3)2 + 2 H2O
When white is ignited with charcoal, calcium metaphosphate yields 2/3rd of white phosphorus and 1/3rd is remain in the residue as calcium orthophosphate:
3 Ca(PO3)2 + 10 C → Ca3(PO4)2 + 10 CO + P
| White Phosphorus VS Red Phosphorus | |
| WHITE PHOSPHORUS | RED PHOSPHORUS |
| Obtained from Phosphate rocks | Obtained by heating White phosphorus (2500 C) |
| Waxy solid | Crystalline solid |
| Poisonous | Non-Poisonous |
| Insoluble in water but soluble in Organic compounds | In solube in water as well as in Organic compounds |
| Highly Reactive | Very Less Reactive |
Phosphorus History
Naming: Greek: phôs (light) & phoros (bearer)
Discovery: Hennig brand (1669), Hamburg (Germany)
Recognised as an element: Antoine Lavoisier (1777)
Phosphorus Uses
White phosphorus is used in flares & military applications (incendiary bombs, smoke bombs, tracer bullets, smoke screening etc..).
Red phosphorus is used in the material (binder, powdered glass, & red phosphorus), which stuck on the sides of matchboxes, and when heat is generated by friction the red phosphorus convert to white phosphorus, which ignites spontaneously.
The largest use of phosphorus compounds (Concentrated phosphoric acids, which may contains 70% – 75% P2O5) are used in fertilizers for agriculture and farm production.
Ammonium phosphate ((NH4)3PO4) is made from phosphate ores, where the ores are firstly converted into phosphoric acids (H3PO4) before being made into ammonium phosphate.
Phosphates (PO43-) are used in the production of special glasses, sodium lamps, in Production of steel, and some other applications, such as pyrotechnics, toothpaste, detergents (phased out in some countries), pesticides etc..
Bone ash (calcium phosphate, Ca5(OH)(PO4)3) is used to create Fine chinaware and to produce mono-calcium phosphate (CaH4P2O8), which is used in Baking powder.
Trisodium phosphate (TSP, Na3PO4) is important as a water softener, as a cleaning agent, and for preventing boiler scale and corrosion of pipes & boiler tubes.
Biological role of Phosphorus
Phosphorus as phosphate is essential to all living things, even it is an essential ingredient of all protoplasm, nervous tissue, and bones.
It forms the sugar-phosphate backbone of DNA & RNA .
It is important for energy distribution in cells as part of Adenosine Triphosphate (ATP), and is found in many other biologically important molecules.
We intake about 800-1000 mg of phosphate per day, and store about 650-750 grams in our bodies, since our bones & teeth are mainly calcium phosphate (Ca3(PO4)2).
But White Phosphorus is very poisonous, even 50 mg is considered an approximate fatal dose.
Exposure to white phosphorus should not exceed 0.1 mg/m3 (8 hour a day time weighted average (TWA), 40 hour work per week).
White phosphorus should be kept in water because it is dangerously reactive in air and should be handled with tongs/forceps, as contact with the skin may cause strongly/severe burns.
Over-use of Phosphate from detergents & fertilizers can cause them to pollute Lakes & Rivers, causing algae to grow rapidly.
The algae block out the Light to stopping further photosynthesis, due to this, Oxygen dissolved in the water soon gets used up and finaly the Lake dies.
When it exposed to sunlight or heated to 250°C, it is converted to the Red form.
This Red form of phosphorus does not ignite spontaneously in air and is also not as dangerous as white phosphorus.
However, it should be handled with care, because it does convert to the white form at some heated temperatures and that time it emits highly toxic fumes of the oxides of phosphorus.
The red form is fairly stable and sublimes with a vapor pressure of 1 atm at 170 C.
| Foods high in Phosphorus | ||
| Food | Quantity | Contain Phosphorus |
| Roasted Chicken | 140 grams | 300 mg |
| Cooked Pork | 85 grams | 175-224 mg |
| Fried Organ meats (Liver, Brain etc..) | 85 grams | 350-385 mg |
| Seafood (cuttlefish, squid & octopus) | 85 grams | 490 mg |
| Carp (fish) | 85 grams | 451 mg |
| Sardines (fish) | 85 grams | 411 mg |
| Pollock (fish) | 85 grams | 410 mg |
| Clams (fish) | 85 grams | 287 mg |
| Scallops (fish) | 85 grams | 284 mg |
| Salmon (fish) | 85 grams | 274 mg |
| Catfish (fish) | 85 grams | 258 mg |
| Mackerel (fish) | 85 grams | 236 mg |
| Carb (fish) | 85 grams | 238 mg |
| Crayfish (fish) | 85 grams | 230 mg |
| Cheese (Dairy) | 28 grams | 213 mg |
| Skim milk (Dairy) | 245 grams | 175-240 mg |
| Roasted Sunflower & Pumpkin seed | 28 grams | 315 mg |
| Nuts (cashews, almonds, pine nuts & pistachious) | 60-70 grams | 280 mg |
| Wheat (whole grains) | 194 grams | 291 mg |
| Oats (whole grains) | 234 grams | 180 mg |
| Rice (whole grains) | 194 grams | 162 mg |
| Amaranth (small seeds) | 246 grams | 364 mg |
| Quinoa (small seeds) | 246 grams | 280 mg |
| Beans | 164-182 grams | 250 mg |
| Boiled Lentils | 198 grams | 357 mg |
| Roasted SoyBeans | 172 grams | 700 mg |
Abundance of Phosphorus
Phosphorus is not found in pure form in nature, but is widely found in compounds (phosphates, PO43-) in minerals.
Phosphate rocks is the important source of the element, which contains the Apatite (Ca5(PO4)3(F,Cl,OH)) minerals.
Some scientist believe that as per our rate of consumption of this element, a “peak phosphorus” will occur in around year 2050, and reserves will be depleted in the next 50 to 90 years.
There are serious concerns that, how long ‘peak phosphorus’ will occur in future, after which our sources will dwindle (decline).
In this case, there could be a serious problem for the worlds food production, Because phosphorus is an essential ingredient in fertilizers.
White phosphorus is commercially manufactured by heating phosphate rock in the presence of carbon & silica in a furnace, where phosphorus produced as a vapour, which is then collected under water.
Red phosphorus is made by slowly heating white phosphorus to about 250°C in the absence of air.

Annual world wide production is around 287 million tonnes, and World wide Reserve is around 70 billion tonnes.
0.0007% (In Universe)
0.11% (In Meteorites)
0.0007% (In Sun)
0.099% (In Earth’s Crust)
7×10-6% (In Oceans)
1.1% (In Humans)
World’s Top 3 producers of Phosphorus
1) China
2) Mexico
3) Morocco
World’s Top 3 Reserve holders of Phosphorus
1) Morocco
2) China
3) USA
Phosphorus Price:
Pure (99.99%) metal price is around $280-$320 per KG (KiloGram)
#phosphorus


