26 Fe (Iron Element)

Iron is a lustrous, malleable, ductile, silver-gray metal.
Wrought iron is also known as Pure Iron contains only a few tenths of a percent of carbon, is tough, less fusible, malleable, and usually has a “fibrous” structure.
The pure metal (Pure Irons) is very reactive chemically and rapidly corrodes or rusts in moist air or at elevated temperatures.
It has four allotropic forms, such as α-alpha, γ-gamma, δ-delta and ε–epsilon, with transition points at 912, 1394, and 1530 oC.
The α-alpha form is magnetic, but when transforming into the γ-gamma form, the magnetism disappears at 770 oC (change ferromagnetic to paramagnetic), although the lattice remains unchanged upto 912 oC.
It dissolves readily in dilute acids, cause of Iron is chemically active and forms two major series of chemical compounds, the bivalent iron (II) or ferrous compounds, and the trivalent iron (III) or ferric compounds.

Identity
CAS Number: CAS7439-89-6
CID Number: CID23925
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSNO4565500
CONTENT INDEX
Basic Properties of Iron
Appearance: lustrous metallic with a grayish tinge
Mass Number: 56
Standard Atomic weight: 55.844 g/mol
Atomic number (Z): 26
Electrons: 26
Protons: 26
Neutrons: 30
Period: 4
Group: 8
Block: d
Element category: transition metal
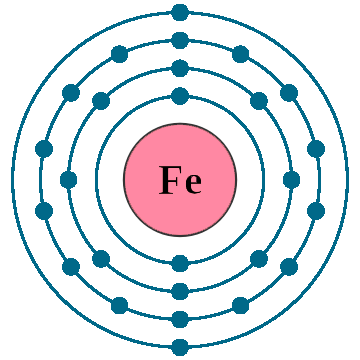
Electrons per shell: K2, L8, M14, N2
Electron configuration: 1s22s22p63s23p63d64s2
Thermal Properties of Iron
Phase: Solid
Melting point: 1811 K (1538 oC, 2800 oF)
Boiling point: 3134 K (2862 oC, 5182 oF)
Debye temperature: 460 K (185.85 oC, 368.33 oF)
Fusion heat: 13.81 kJ/mol
Vaporization heat: 340 kJ/mol
Specific heat: 449 J/(kg K)
Molar heat capacity: 25.11 J/(mol.K)
Thermal expansion: 11.8 μm/(m∙K)
Thermal conductivity: 80.4 W/(m∙K)
Electrical properties of Iron
Electrical conductivity: 10×106 S/m
A Electrical resistivity: 96.2 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Iron
Magnetic type: ferromagnetic
Curie point: 1043 K (769.85 oC, 1417.73 oF) (above which Ferromagnetism vanishes)
Physical Properties of Iron
Density: 7.875 g/cm3 (In solid) 6.97 g/cm3 (In Liquid at M.P)
Molar volume: 0.0000070923 m3/mol
Young’s modulus: 210 GPa
Shear modulus: 82 GPa
Mohs Hardness: 4.0
Bulk modulus: 170 GPa
Poisson ratio: 0.29
Vicker hardness: 608 MPa
Brinell hardness: 200-1180 MPa (490 MPa)
Sound Speed: 5120 m/s
Atomic Properties of Iron
Oxidation states: -4, -2, -1, +1, +2, +3, +4, +5, +6, +7
Valence Electrons: 3d6 4s2
Ion charge: Fe3+ Fe2+
Ionization potential of an atom: 7.83
Ionization energies: 1st: 762.5 kJ.mol 2nd: 1562 kJ/mol 3rd: 2956.9 kJ/mol
Ionic radius: 645 pm
Atomic radius: 126 pm (empirical)
Van der Waals: 194 Pm
Covalent radius: 132±8 pm (Low spin), 152±6 pm (High spin)
Filling Orbital: 3d6
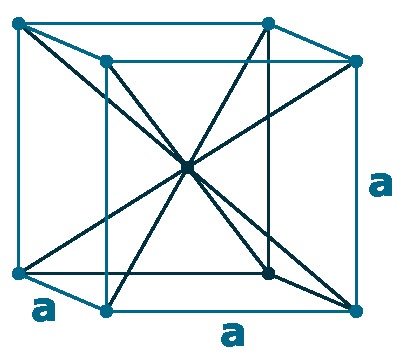
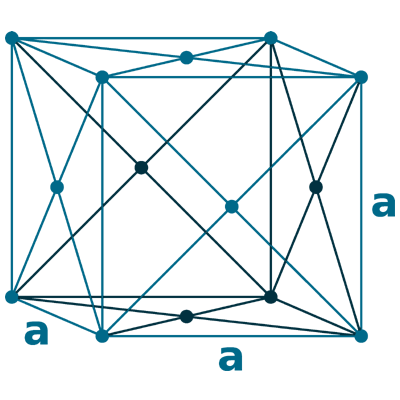
Crystal structure: Body centered cubic (δ-allotrope, At 1538 oC), Face centered cubuc (γ-allotrope, At 1394 oC)
Lattice angles: π/2, π/2, π/2
Lattice constant: 286.6, 286.6, 286.6 pm
Grid parameters: a=2.866 Å
Space Group Name: lm_3m
Space Group Number: 229
Reactivity of Iron
Electronegativity: 1.83 (pauling scale)
Valence: +3
Electron affinity: 15.7 kJ/mol
Nuclear Properties of Iron
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 5D4
Neutron cross section (Brans): 2.56
Neutron Mass Absorption: 0.0015
Isotopes: 54Fe 55Fe 56Fe 57Fe 58Fe 59Fe 60Fe
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 54Fe | 5.85 | 53.941 | Stable |
| 55Fe | Syn | – | 2.72 y |
| 56Fe | 91.75 | 55.935 | Stable |
| 57Fe | 2.12 | 56.935 | Stable |
| 58Fe | 0.28 | 57.934 | Stable |
| 59Fe | Syn | – | 44.5 d |
| 60Fe | Trace | – | 2.6×106 y |
Chemical Reactions of Iron
Reacts with oxygen O2, and forming Fe(II) and Fe(III) oxides:
4 Fe (s) + 3 O2 (g) → 2 Fe2O3 (s)
3 Fe (s) + 2 O2 (g) → Fe3O4 (s)
Reacts with water:
Fe3+ (aq) + 4 H2O (I) ⇌ FeO42- (aq) + 8 H+ (aq) + 3e–, Eo = -2.20 V
AIron reacts with excess of Fulorine, Chlorine and Bromine to form ferric, that is Fe(III) halides:
2 Fe (s) + 3 F2 (g) → 2 FeF3 (s) [white]
2 Fe (s) + 3 Cl2 (g) → 2 FeCl3 (s) [dark brown]
2 Fe (s) + 3 Br2 (I) → 2 FeBr3 (s) [reddish brown]
AIron(III) is too oxidizing and the iodide is too reducing, and above reaction is not successful for iodine, so the direct reaction between iron metal and iodine can be used to form Iron (II) iodide:
Fe (s) + I2 (s) → FeI2 (s) [grey]
Fe(III) forms yellow chloro complexes, and the complexed are readily hydrolyzed by heat. where, Fe (III) is reduced to Fe (II) by I–:
2 Fe3+ (aq) + 2 I– (aq) → 2 Fe2+ (aq) + I2 (aq)
The metal dissolves readily in dilute sulphuric acid (H2SO4) in the absence of oxygen, and forming Fe(II) ions and H2. In this aqueous solution, Fe(II) is present as the complex [Fe(H2O)6]2+.
Fe (s) + H2SO4 (aq) → Fe2+ (aq) + SO42- (aq) + H2 (g)
If oxygen is present, then some of the Fe (II) oxidizes to Fe (III).
3 Fe (s) + 4 H2SO4 (aq) → Fe2+ (aq) + 2 Fe3+ (aq) + 4 SO42- (aq) + 4 H2 (aq)
Concentrated HNO3 (nitric acid) reacts on the surface of iron, and passivates the surface.
Iron(III) chloride reacts with a phenol, and form a deep violet complex:
3 ArOH + FeCl3 → Fe(OAr)3 + 3 HCl (Ar (aryl) = aromatic ring)
Iron History
Naming: Latin, ferrum; Anglo-Saxon, iron
Discovery: Before 5000 BC
Iron Uses
AIron is the most important of all metals, and about 90% of all metal that is refined today is iron.
Most of Iron is used to manufacture steel, which is used in civil engineering (reinforced concrete, girders etc..) and in manufacturing.
The pure metal (wrought iron) is not often encountered in commerce, but is usually alloyed with carbon or other metals.
Steel is the best known alloy of iron, and some of the forms that iron takes include: pig iron, cast iron, wrought iron, alloy steels, carbon steel, iron oxides.
There are many different types of steel are use with different properties, Like ordinary carbon steel is an alloy of iron with carbon (contains 0.05%-0.25% in Low carbon steel, 0.29%-0.54% in Medium carbon steel, and 0.55%-2.1% High carbon steel), and with small amounts of other elements.
Alloy steels with carbon and other additives (such as nickel, tungsten, vanadium, chromium, and manganese), are stronger and tougher than carbon steels and have a huge variety of applications including bridges, bicycle chains, electricity pylons, cutting tools, rifle barrels etc..
Stainless steel is very resistant to corrosion, It contains at least 16-26% chromium, 6-22% nickel (Increase corrosion resistance), and Other metals such as titanium, molybdenum, and copper are added to enhance its strength and workability.
It is used in architecture, cutlery, bearings, surgical instruments, jewellery etc..
Cast iron (contains 3–5% carbon) is used for valves, pipes, pumps etc..
It is not tough as steel but it is hard and cheaper.
Magnets are made up of iron and its alloys and compounds.
Iron catalysts are used in the Haber process (producing ammonia from nitrogen and hydrogen), and in the Fischer–Tropsch process (converts a mixture of hydrogen and carbon monoxide into liquid hydrocarbons).
Biological role of Iron
AIron is non-toxic, even it is an essential elements for all Life, where, average human contains about 4gm of Iron.
TheIron is an essential part of hemoglobin (the red colouring agent of the blood), that carries oxygen from our lungs to the cells, where it is needed for tissue respiration (tansport oxygen through our body).
Humans need 10–18 mg (milligrams) airon each day, Because a lack of iron will cause anaemia to develop.
The human body absorbs iron in animal products faster than iron in plant products, Foods such as liver, kidney, molasses (black treacle), brewer’s yeast, cocoa and liquorice contain a lot of iron.
Anemia (A condition in which the blood doesn’t have enough healthy red blood cells) is more common problem is occur in human, due to iron deficiency.
Abundance of Iron
AIron is a relatively abundant element in the universe, and the 4th most abundant element in the Earth’s crust, and the core of the Earth has largely composed of Iron with Nickel and Sulfur.
The most common iron ore is Haematite (ferric oxide, Fe2O3), but iron is found widely in other minerals such as magnetite (Iron oxide, Fe3O4), Lepidocrocite (FeO(OH)), taconite (upto 30% Fe), and Siderite (Iron carbonate, FeCO3).
Commercially, aIron is produced in a blast furnace by heating haematite or magnetite with coke (high-carbon fuel) and limestone (calcium carbonate, CaCO3).
This process forms pig Iron, which contains about 3% of carbon and other impurities (sulfur, silicon, manganese and phosphorus), and this pig Iron is further used to make steel.
Annual world wide production of crude steel is around 1,300,00,000 tons (1.3 Billion)
0.11% (In Universe)
22% (In Meteorites)
0.1% (In Sun)
6.3% (In Earth’s Crust)
3×10-7% (In Oceans)
0.006% (In Humans)




World’s Top 3 producers of Iron
1) China
2) Australia
3) Brazil
World’s Top 3 Reserve holders of Iron
1) Australia
2) Brazil
3) Russia
#Iron


