22 Ti (Titanium Element)

Pure Titanium is a lustrous, white-silvery metal, and has a low density, good strength, easily fabricated, and an excellent corrosion resistance.
It is ductile only in the oxygen-free environment.
It resistant to tarnishing in air at room temperature, but exposed to elevated temperatures in air and form a passive but protective oxide coating (leading to corrosion-resistance).
The metal, which burns in the air as well as in nitrogen, and resistant to dilute sulfuric (H2SO4) and hydrochloric acid (HCl), most organic acids, most chlorine gas, and chloride solutions.
Pure metal is not soluble in water but is soluble in concentrated acids.
Natural titanium is reported to become very radioactive after bombardment with deuterons (the nucleus of a deuterium atom).
The emitted radiations are mostly positrons (positive electron) and hard gamma rays.
The metal is dimorphic, where the hexagonal alpha form changes to the cubic beta form very slowly at about 880°C, and the metal combines with oxygen at red heat, and with chlorine at 550°C.
Titanium di-oxide (TiO2) is relatively clear and has an extremely high index of refraction with an optical dispersion higher than diamond.

Identity
CAS Number: CAS7440-19-9
CID Number: CID23951
DOT Hazard Class: 4.2
DOT Number: 2546
CONTENT INDEX
Basic Properties of Titanium
Pronunciation: Ta-tay-nee-am
Appearance: Silvery grey-white Metallic
Mass Number: 48
Standard Atomic weight: 47.867 g/mol
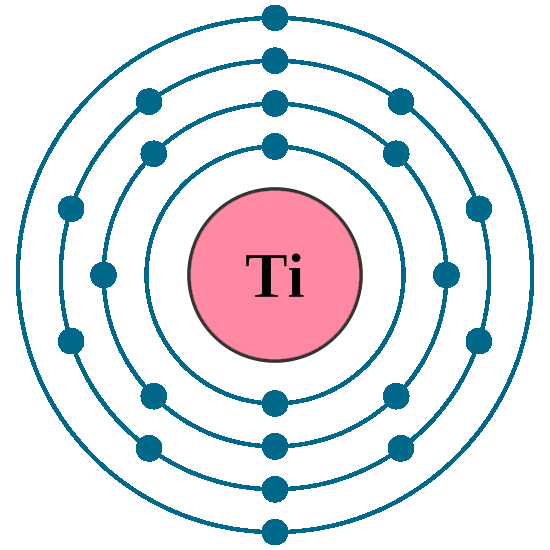
Atomic number (Z): 22
Electrons: 22
Protons: 22
Neutrons: 26
Period: 4
Group: 4
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M10, N2
Electron configuration: 1s22s22p63s23p63d24s2
Thermal Properties of Titanium
Phase: Solid
Melting point: 1941 K (1668 oC, 3034 oF)
Boiling point: 3560 K (3287 oC, 5949 oF)
Debye temperature: 308 K (106.85 oC, 224.33 oF)
Fusion heat: 14.15 kJ/mol
Vaporization heat: 425 kJ/mol
Specific heat: 520 J/(kg K)
Molar heat capacity: 25.060 J/(mol.K)
Thermal expansion: 8.6 μm/(m∙K)
Thermal conductivity: 22 W/(m∙K)
Electrical properties of Titanium
Electrical conductivity: 2.5×106 S/m
A Electrical resistivity: 420 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.4 K (-272.75 oC, -458.95 oF)
Magnetic Properties of Titanium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +153×10-6 cm3/mol
Volume magnetic susceptibility: 0.0001807
Mass magnetic susceptibility: 40.1×10-9 m3/kg
Molar magnetic susceptibility: 1.919×10-9 m3/mol
Physical Properties of Titanium
Density: 4.505 g/cm3 (In solid) 4.10 g/cm3 (In Liquid at M.P)
Molar volume: 0.000010621 m3/mol
Young’s modulus: 116 GPa
Shear modulus: 44 GPa
Mohs Hardness: 6.0
Bulk modulus: 110 GPa
Poisson ratio: 0.32
Vicker hardness: 830-3420 MPa
Brinell hardness: 715-2770 MPa
Sound Speed: 5090 m/s
Atomic Properties of Titanium
Oxidation states: -2, -1, 1, 2, 3, 4
Valence Electrons: 3d2 4s2
Ion charge: Ti4+ Ti3+
Ionization potential of an atom: 6.81
Ionization energies: 1st: 659 kJ.mol 2nd: 1310 kJ/mol 3rd: 2652.5 kJ/mol
Ionic radius: 60.5 pm
Atomic radius: 147 pm (empirical)
Van der Waals: 187 Pm
Covalent radius: 160±8 pm
Filling Orbital: 3d2
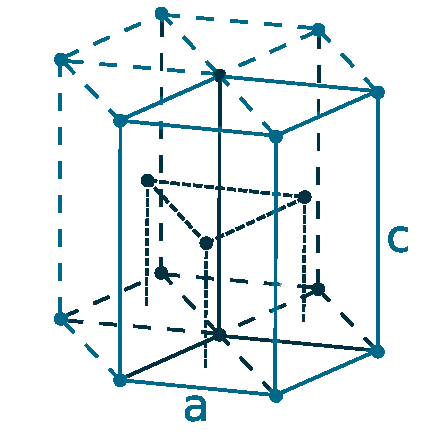
Crystal structure: Hexagonal close packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 295.1, 295.1, 469.7 pm
Grid parameters: a=2.951 Å c=4.697 Å
Attitude c/a: 1.587
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Titanium
Electronegativity: 1.54 (pauling scale)
Valence: +4
Electron affinity: 7.6 kJ/mol
Nuclear Properties of Titanium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3F2
Neutron cross section (Brans): 6.1
Neutron Mass Absorption: 0.0044
Isotopes: 44Ti 46Ti 47Ti 48Ti 49Ti 50Ti
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 44Ti | Syn | – | 63 y |
| 46Ti | 8.25 | 45.954 | Stable |
| 47Ti | 7.44 | 46.953 | Stable |
| 48Ti | 73.72 | 47.948 | Stable |
| 49Ti | 5.41 | 48.948 | Stable |
| 50Ti | 5.18 | 49.945 | Stable |
Chemical Reactions of Titanium
The metal doesn’t react with air under normal condition, and when it start to burn, it reacts with both oxygen and nitrongen to form:
Ti (s) + O2 (g) → TiO2 (s) [White] (Titanium oxide)
2 Ti (s) + N2 (g) → 2 TiN (s) (Titanium nitride)
It doesn’t reacts with water under normal condition, but reacts when the water is heated to steam, and forming titanium (IV) Oxide (TiO2) and Hydrogen gas.
Ti (s) + 2 H2O (g) → TiO2 [White] + 2 H2 (g)
Reacts with Halogens, when heated, and forms titanium(IV) halides:
Ti (s) + 2 F2 (g) → TiF4 (s) [white] (Titanium (IV) fluoride)
Ti (s) + 2 Cl2 (g) → TiCl4 (s) [colourless] (Titanium (IV) chloride)
Ti (s) + 2 Br2 (g) → TiBr4 (s) [orange] (Titanium (IV) Bromide)
Ti (s) + 2 I2 (g) → TiI4 (s) [dark brown] (Titanium (IV) iodide)
Titanium reacts with dilute aqueous Hydrofluoric acid (Hf), and forms the complex anion [TiF6]3- with hydrogen.
2 Ti (s) + 12 HF (aq) → 2 [TiF6]3- (aq) + 3 H2 (g) + 6 H+ (aq)
It doesn’t react with mineral acids under normal condition, but does react with hot hydrochloric acid to form titanium (III) complexes:
2 Ti (s) + 12 HCl (aq) → 2 Ti[Cl6]3- (aq) + 3 H2 (g) + 6 H+ (aq)
Production
Common titanium alloys are made by reduction, For example, cuprotitanium, manganotitanium, and ferrocarbon titanium:
2 FeTiO3 + 7 Cl2 + 6 C → 2 TiCl4 + 2 FeCl3 + 6 CO (900 °C)
TiCl4 + 2 Mg → 2 MgCl2 + Ti (1,100 °C)
Titanium History
Naming: Greek mythology: titanos (Titans), the sons of the Earth goddess.
Naming by: Martin Heinrich Klaproth (1795)
Discovery: William Gregor (1791)
First isolation: Jons Jakob Berzelius (1825)
Titanium Uses
ATitanium is as strong as steel but much less dense (45% lighter), and twice as strong as aluminum but 60% heavier, therefore it is important as an alloying agent with many metals including Iron, Aluminum, Manganese, Molybdenum and other metals.
Titanium alloys are mainly used Armour plating, Naval ships, Spacecraft, Aircraft, and Missiles because of their low density, light weight, very high tensile strength and ability to withstand extremes of temperature.
It is also used in bicycles, laptops, golf clubs, and crutches (a long stick with a crosspiece, used as a support under the armpit by a lame person).
It also has an excellent corrosion resistance property, even in seawater, so it used in condenser pipes of power plant, in desalination plants (converting sea water into fresh water) and to protect the hulls (main body of a ship) of ships, submarines and other structures exposed to seawater.
In Medical, titanium metal connects well with bone, so it has found surgical applications such as in hip and knee replacements, pace-makers (used to treat arrhythmias), bone-plates and screws and cranial plates for skull fractures, and also used in tooth implants.
The largest use of titanium is in the form of titanium di-oxide (TiO2), which is extensively used as a white pigment in artists paint, house paint, plastics, paper, and porcelain enamels to giving them a final touch of great brightness, hardness, and acid resistance.
A typical (distinctive qualities) Lipstick contains 10% titanium.
TiO2 has a great coating power, and also a good reflector of infrared radiation, so it is used in solar observatories where heat causes poor visibility.
It is also used in sunscreens because it prevents UV light from reaching the skin, even Its Nanoparticles appear invisible when applied to the skin.
Titanium tetra-chloride (TiCl4) is used to iridize glass, and this compound fumes strongly in air and has been used to produce smoke screens.
Biological role of Titanium
It is non-toxic, but Fine titanium di-oxide dust is a suspected carcinogen (a substance capable of causing cancer).
Abundance of Titanium
Titanium is the 9th most abundant element in the Earth crust, and almost always present in igneous rocks (magmatic rock).
It occurs in the minerals Rutile (TiO2), ilmenite (FeTiO3), and Sphene (Titanite, CaTiSiO₅) and is present in many iron ores.
It is also present in plants, in the ash of coal, and in the human body.
Commercially, Titanium is produced by reducing titanium(IV) chloride (TiCl4) with magnesium, and The metal can be purified by decomposing the iodide, This method is still largely used for producing the metal,
Titanium(IV) oxide (TiO2) is produced by either the ‘chloride process’ or the ‘sulfate process’, both of which use the mineral ilmenite (FeTiO3) as a starting material.
Annual world wide production is around 2,20,000 tons, and World wide Reserve is around 600,100,000 tons.
0.0003% (In Universe)
0.054% (In Meteorites)
0.0004% (In Sun)
0.66% (In Earth’s Crust)
1×10-7% (In Oceans)



World’s Top 3 producers of Titanium
1) Canada
2) Australia
3) South Africa
World’s Top 3 Reserve holders of Titanium
1) China
2) Australia
3) India
#Titanium