43 Tc (Technetium)

Technetium is a silvery-gray metal that tarnishes slowly in moist air.
It is dissolves in Nitric acid, Aqua regia (mixture of nitric acid and hydrochloric acid), and conc. sulfuric acid (H2SO4), is insoluble in hydrochloric acid (HCl) of any strength.
Under oxidizing conditions technetium (VII) will exist as the pertechnetate ion, TcO4-.
The metal is an excellent superconductor at 7.4 K and below. Below this temperature, technetium has a very high magnetic penetration depth, greater than any other element except niobium.

Identity
CAS Number: CAS7440-26-8
CONTENT INDEX
Basic Properties of Technetium
Pronunciation: Pek-nee-shee-am
Appearance: Shiny gray Metal
Mass Number: 98
Atomic weight: 98.906 g/mol
Atomic number (Z): 43
Electrons: 43
Protons: 43
Neutrons: 55
Period: 5
Group: 7
Block: d
Element category: Transition metal
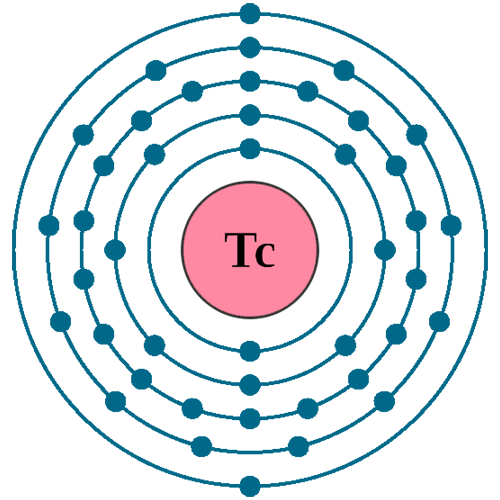
Electrons per shell: K2, L8, M18, N13, O2
Electron configuration: 1s22s22p63s23p63d104s24p64d55s2
Thermal Properties of Technetium
Phase: Solid
Melting point: 2430 K (2157 oC, 3915 oF)
Boiling point: 4538 K (4265 oC, 7709 oF)
Fusion heat: 33.29 kJ/mol
Vaporization heat: 585.2 kJ/mol
Specific heat: 63 J/(kg K)
Molar heat capacity: 24.27 J/(mol.K)
Thermal expansion: 7.1 μm/(m∙K)
Thermal conductivity: 50.6 W/(m∙K)
Electrical properties of Technetium
Electrical conductivity: 5×106 S/m
A Electrical resistivity: 200 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 7.8 K (-265.35 oC, -445.63 oF)
Magnetic Properties of Technetium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +270×10-6 cm3/mol
Volume magnetic susceptibility: 0.0003933
Mass magnetic susceptibility: 34.2×10-9 m3/kg
Molar magnetic susceptibility: 3.352×10-9 m3/mol
Physical Properties of Technetium
Density: 11 g/cm3 (In solid)
Molar volume: 0.000008434 m3/mol
Sound Speed: 16,200 m/s
Atomic Properties of Technetium
Oxidation states: 7, 6, 5, 4, 3, 2, 1, -1, -3
Valence Electrons: 4d6 5s1
Ion charge: Tc7+
Ionization energies: 1st: 702 kJ.mol 2nd: 1470 kJ/mol 3rd: 2850 kJ/mol
Ionic radius: 56 pm
Atomic radius: empirical: 136 pm
Van der Waals: 209 pm
Covalent radius: 147±7 pm
Filling Orbital: 4d6
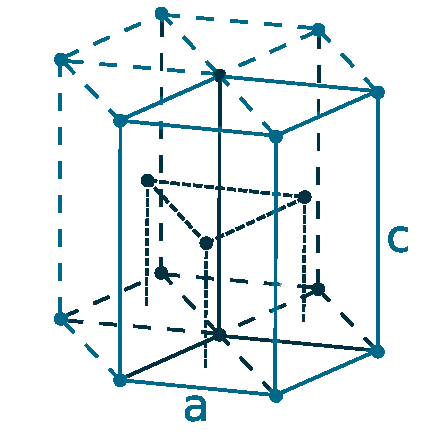
Crystal structure: Hexagonal close-packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 273.5, 273.5, 438.8 pm
Grid parameters: a=2.735 Å c=4.388 Å
Attitude c/a: 1.602
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Technetium
Electronegativity: pauling scale: 1.9
Valence: 6
Electron affinity: 53 kJ/mol
Nuclear Properties of Technetium
Half Life: 211098 y
Lifetime: 6.024 y
Decay mode: β-Beta Decay
Quantum Number: 6S5/2
Neutron cross section (Brans): 22
Isotopes: 95mTc 96Tc 97Tc 97mTc 98Tc 99Tc 99mTc
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 95mTc | Syn | – | 61 d |
| 96Tc | Syn | – | 4.3 d |
| 97Tc | Syn | 96.906 | 2.6×106 y |
| 97mTc | Syn | – | 91 d |
| 98Tc | Syn | 97.907 | 4.2×106 y |
| 99Tc | Trace | 98.906 | 2.11×105y |
| 99mTc | Syn | – | 6.01 h |
Chemical Reactions of Technetium
Technetium tarnishes very slowly in moist air, and If heating with oxygen then Forming technetium heptoxide:
4 Tc (s) + 7 O2 (g) → 2 Tc2O7 (s) (technetium (VII) oxide)
Reacts With Halogen:
When Technetium heated with fluorine, forming a mixture of Technetium hexafluoride and Technetium heptafluoride:
Tc (s) + 3 F2 (g) → TcF6 (s) (Technetium (VI) fluoride)
2 Tc (s) + 7 F2 (g) → 2 TcF7 (s) (Technetium (VII) fluoride)
Reacts with water:
Tc (s) + 2 H2O (l) ⇌ TcO2 (s) + 4 H+ (aq) + 4 e– Eo = -0.272 V
TcO2 (s) + H2O (l) ⇌ TcO3 (s) + 2 H+ (aq) + 2 e– Eo = -0.757 V
Tc2+ (aq) + 4 H2O (l) ⇌ TcO4– (aq) + 8 H+ (aq) + 5 e– Eo = -0.500 V
Technetium History
Naming: Greek: technêtos (artificial).
Prediction: Dmitri Mendeleev (1871)
Discovery and first isolation: Emilio Segre and Carlo perrier (1937) in Palermo Italy (Sicily)
Technetium Uses
gamma-ray (140 keV) emitting Technetium-99m (“m”, metastable) is most useful isotope, which is widely used for medical diagnostic studies.
Because of Its half-life being short (T1/2 = 2.1×105 year), It binds chemically to many biologically active molecules, these properties make it suitable for many medical radioactive isotope tests.
Several chemical forms are used to image different parts of the body (The sulfur colloid of Isotope 99mTc is scavenged by the spleen, making it possible to image the structure of the body).
Technetium is a remarkable corrosion resistor for steel, and adding very small amounts can provide excellent protection from corrossion.
Technetium-99 Isotope has also been proposed for use in optoelectronic devices and nano-scale nuclear batteries
atechnetium is far more effective catalyst than either rhenium or palladium.
Biological role: It is toxic due to its radioactivity. 99Tc is a contamination hazard and it should be handled with care.
Abundance of Technetium
It is obtained as a grey powder.
The metal is produced in tonnes quantities from the fission products of uranium nuclear fuel.
A kilogram of uranium contains an estimated 1 nanogram (10-9 g) of atechnetium.
Annual world wide production is in Kilograms.
#technetium