44 Ru (Ruthenium)

Ruthenium is an element of Platinum group metals (PGM) together with Rhodium, palladium, osmium, iridium, and platinum.
Ruthenium is a hard, white metal.
It does not tarnish in air at room temperatures, and is not attacked by hot or cold acids or aqua regia, but It oxidizes explosively (when potassium chlorate is added to the solution).
It is attacked by halogens, hydroxides, etc and dissolved in molten alkalis.
Ruthenium can be plated by electrodeposition or by thermal decomposition methods.

Identity
CAS Number: CAS7440-18-8
CID Number: CID23950
DOT Hazard Class: 4.1
DOT Number: 3089
CONTENT INDEX
Basic Properties of Ruthenium
Pronunciation: Roo-thee-nee-am
Appearance: silvery white Metallic
Mass Number: 101
Standard Atomic weight: 101.07 g/mol
Atomic number (Z): 44
Electrons: 44
Protons: 44
Neutrons: 57
Period: 5
Group: 8
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M18, N15, O1
Electron configuration: 1s22s22p63s23p63d104s24p64d75s1

Thermal Properties of Ruthenium
Phase: Solid
Melting point: 2607 K (2334 oC, 4233 oF)
Boiling point: 4423 K (4150 oC, 7502 oF)
Debye temperature: 600 K (326.85 oC, 620.33 oF)
Fusion heat: 38.59 kJ/mol
Vaporization heat: 619 kJ/mol
Specific heat: 238 J/(kg K)
Molar heat capacity: 24.06 J/(mol.K)
Thermal expansion: 6.4 μm/(m∙K)
Thermal conductivity: 117 W/(m∙K)
Electrical properties of Ruthenium
Electrical conductivity: 14×106 S/m
A Electrical resistivity: 71 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.49 K (-272.66 oC, -458.788 oF)
Magnetic Properties of Ruthenium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +43.2×10-6 cm3/mol
Volume magnetic susceptibility: 0.000067
Mass magnetic susceptibility: 5.42×10-9 m3/kg
Molar magnetic susceptibility: 0.54×10-9 m3/mol
Physical Properties of Ruthenium
Density: 12.45 g/cm3 (In solid) 10.65 g/cm3 (In Liquid at MP)
Molar volume: 0.0000081706 m3/mol
Young’s modulus: 447 GPa
Shear modulus: 173 GPa
Mohs Hardness: 6.5
Bulk modulus: 220 GPa
Poisson ratio: 0.30
Vickers Hardnes: 2298.138 MPa
Brinell hardness: 2160 MPa
Sound Speed: 5970 m/s
Atomic Properties of Ruthenium
Oxidation states: -4, -2, 1, 2, 3, 4, 5, 6, 7, 8
Valence Electrons: 4d7 5s1
Ion charge: Ru3+ Ru4+
The ionization potential of an atom: 7.5
Ionization energies: 1st: 710.2 kJ.mol 2nd: 1620 kJ/mol 3rd: 2747 kJ/mol
Ionic radius: 68 pm
Atomic radius: empirical: 134 pm
Van der Waals: 207
Covalent radius: 146±7 pm
Filling Orbital: 4d7
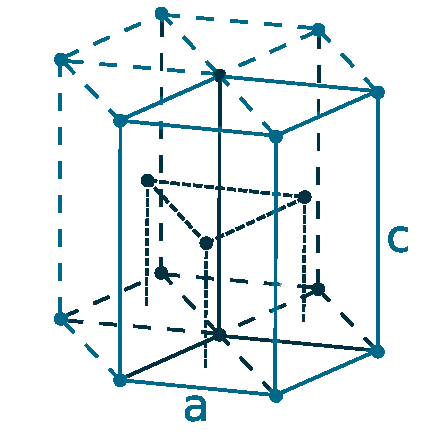
Crystal structure: Hexagonal close-packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 270.59, 270.59, 270.59 pm
Grid parameters: a=2.706 Å c=4.282 Å
Attitude c/a: 1.582
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Ruthenium
Electronegativity: pauling scale: 2.2
Valence: +6
Electron affinity: 101.3 kJ/mol
Nuclear Properties of Ruthenium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 5F5
Neutron cross section (Brans): 2.6
Neutron Mass Absorption: 0.0009
Isotopes: 96Ru 97Ru 98Ru 99Ru 100Ru 101Ru 102Ru 103Ru 104Ru 106Ru
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 96Ru | 5.54 | 95.908 | Stable |
| 97Ru | Syn | – | 2.9 d |
| 98Ru | 1.87 | 97.905 | Stable |
| 99Ru | 12.76 | 98.906 | Stable |
| 100Ru | 12.60 | 99.904 | Stable |
| 101Ru | 17.06 | 100.906 | Stable |
| 102Ru | 31.55 | 101.904 | Stable |
| 103Ru | Syn | – | 39.26 d |
| 104Ru | 18.62 | 103.905 | Stable |
| 105Ru | Syn | – | 373.59 d |
Chemical Reactions of Ruthenium
Ruthenium is largely immune to atmospheric attack under normal conditions, But If heated then it’s oxidized to:
Ru (s) + O2 (g) → RuO2 (s) (Ruthenium (IV) oxide)
The metal reacts with Halogens:
React with excess of Fluorine:
Ru (s) + 3 F2 (g) → RuF6 (s) [dark brown] (Ruthenium (VI) fluoride)
Heating ruthenium metal to 330 oC with chlorine, in presence of carbon monoxide:
2 Ru (s) + 3 Cl2 (g) → 2 RuCl3 (s) [dark brown] (Ruthenium (III) chloride)
Further heating of this material in the presence of Cl2, gives a Black form of Ru (III) chloride.
The metal reacts with water:
Ru2+ (aq) + 2 H2O (l) ⇌ RuO2 (s) + 4 H+ (aq) + 2 e– Eo = -1.120 V
RuO2 (s) + 2 H2O (l) ⇌ RuO4 (s) + 4 H+ (aq) + 4 e– Eo = -1.387 V
Ru2+ (aq) + 4 H2O (l) ⇌ RuO42- (aq) + 8 H+ (aq) + 4 e– Eo = -1.563 V
Ru2+ (aq) + 4 H2O (l) ⇌ RuO4– (aq) + 8 H+ (aq) + 5 e– Eo = -1.368 V
Ruthenium History
Naming: After Ruthenia (Latin for: medieval Kievan Rus’ region)
Discovery and First isolation: Karl Ernst Claus (1844)
Ruthenium Uses
Ruthenium demand is rising. It is used in the electronics industry (50%) for chip resistors and electrical contacts and in the chemical industry (40%), Ruthenium oxide is used to coat the anodes of electrochemical cells for chlorine production.
Ruthenium is also versatile catalysts, which is used in the removal of H2S from industrial processes, for the production of ammonia from natural gas, and acetic acid from methanol.
Ruthenium is one of the most effective hardeners for platinum and palladium and it is added in small amounts (0.1 %) to improves the corrosion resistance of Titanium.
It is used in electrical contact alloys and filaments, in pen nibs, in jewelry (as an alloy with platinum), and in instrument pivots.
It is also used in alloys with molybdenum (ruthenium-molybdenum alloy is said to be superconductive at 10.6 K), cobalt, tungsten, nickel, and other metals.
Ruthenium compounds can be used in solar cells( which turn light energy into electrical energy), and to color ceramics and glass.
Biological role: Ruthenium(IV) oxide (Ruthenium tetroxide) is highly toxic.
Abundance of Ruthenium
Ruthenium is one of the rarest metals on Earth, and it occurs native with other members of the platinum group, more commonly found with other platinum metals in the minerals pentlandite (in Sudbury, Canada) and pyroxenite (in South africa).
It is obtained commercially from the wastes of nickel refining.
Annual world wide production is around 12 tons.
4×10-7% (In Universe)
8.1×10-5% (In Meteorites)
5×10-7% (In Sun)
1×10-7% (In Earth’s Crust)
7×10-11% (In Oceans)


World’s Top 3 producers of Ruthenium
1) South Africa
2) Russia
3) Zimbabwe
World’s Top 3 Reserve holders of Ruthenium
1) South Africa
2) Russia
3) USA
#Ruthenium


