73 Ta (Tantalum)

Pure tantalum is soft, shiny, silvery metal, and impure metal is gray, heavy, and very hard.
Tantalum is resistant to corrosion due to an oxide film on its surface, which is stable and also have good rectifying and dielectric properties.
It is almost immune to chemical attack at temperatures below 150 oC, and is attacked only by acidic solutions containing the fluoride ion, hydrofluoric acid (HF), and free sulfur trioxide (SO3).
It is much more reactive at high temperature.

Identity
CAS Number: CAS7440-25-7
CID Number: CID23956
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSWW5505000
CONTENT INDEX
Basic Properties of Tantalum
Pronunciation: Tan-tal-am
Appearance: gray blue
Mass Number: 181
Standard Atomic weight: 180.947 g/mol
Atomic number (Z): 73
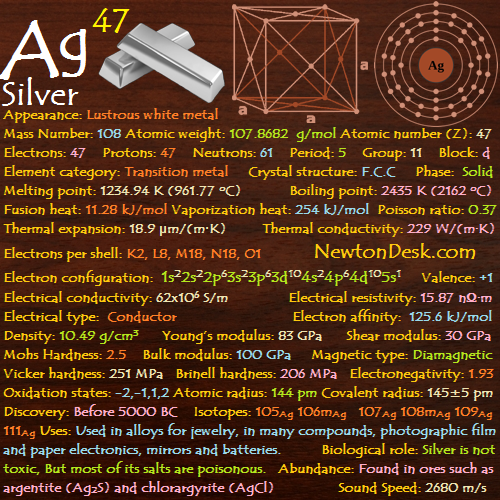
Electrons: 73
Protons: 73
Neutrons: 108
Period: 6
Group: 5
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M18, N32, O11, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f145d36s2
Thermal Properties of Tantalum
Phase: Solid
Melting point: 3290 K (3017 oC, 5463 oF)
Boiling point: 5731 K (5458 oC, 9856 oF)
Debye temperature: 225 K (-48.15 oC, -54.67 oF)
Fusion heat: 36.57 kJ/mol
Vaporization heat: 753 kJ/mol
Specific heat: 140 J/(kg K)
Molar heat capacity: 25.36 J/(mol.K)
Thermal expansion: 6.3 μm/(m∙K)
Thermal conductivity: 57.5 W/(m∙K)
Electrical properties of Tantalum
Electrical conductivity: 7.7×106 S/m
A Electrical resistivity: 131 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 4.47 K (-268 oC, -451 oF)
Magnetic Properties of Tantalum
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +154×10-6 cm3/mol
Volume magnetic susceptibility: 0.0001782
Mass magnetic susceptibility: 10.7×10-9 m3/kg
Molar magnetic susceptibility: 1.936×10-9 m3/mol
Physical Properties of Tantalum
Density: 16.70 g/cm3 (In solid) 15 g/cm3 (In Liquid at M.P)
Molar volume: 0.0000108675 m3/mol
Young’s modulus: 186 GPa
Shear modulus: 69 GPa
Mohs Hardness: 6.5
Bulk modulus: 200 GPa
Poisson ratio: 0.34
Vicker hardness: 870-1200 MPa
Brinell hardness: 450-3430MPa
Sound Speed: 3400 m/s
Atomic Properties of Tantalum
Oxidation states: -3, -1, 1, 2, 3, 4, 5
Valence Electrons: 5d3 6s2
Ion charge: Ta5+
Ionization energies: 1st: 760 kJ.mol 2nd: 1500 kJ/mol
Ionic radius: 64 pm
Atomic radius: 146 pm (empirical)
Van der Waals: 217 Pm
Covalent radius: 170±8 pm
Filling Orbital: 5d3
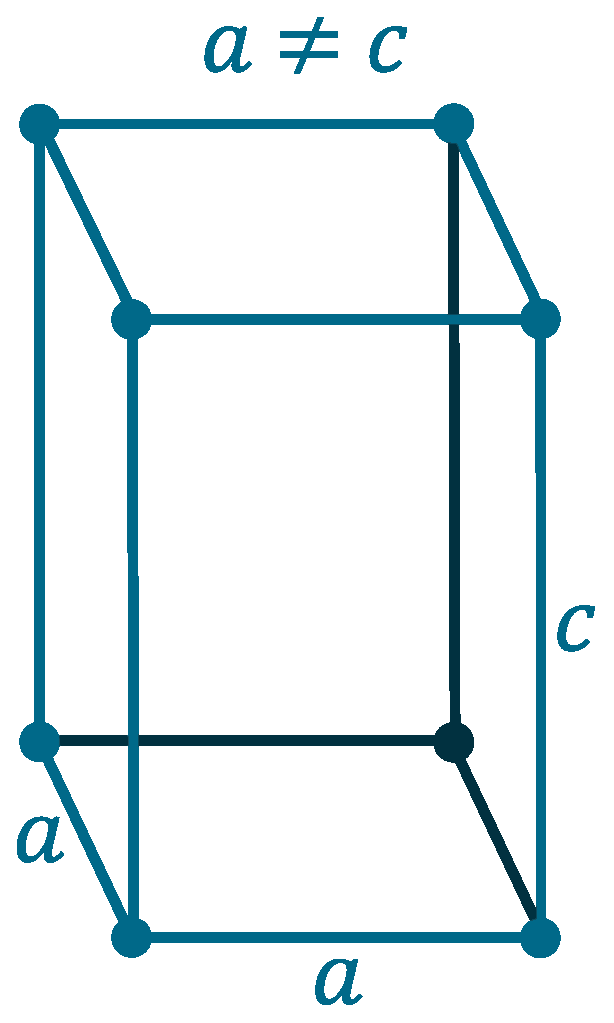
Crystal structure: Body centered cubic (for α-Ta), Tetragonal (for β-Ta)
Lattice angles: π/2, π/2, π/2
Lattice constant: 330.13, 330.13, 330.13 pm
Grid parameters: a=3.310 Å
Space Group Name: lm_3m
Space Group Number: 229

Reactivity of Tantalum
Electronegativity: 1.5 (pauling scale)
Valence: +5
Electron affinity: 31 kJ/mol
Nuclear Properties of Tantalum
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 4F3/2
Neutron cross section (Brans): 20.5
Neutron Mass Absorption: 0.0041
Isotopes: 177Ta 178Ta 179Ta 180Ta 180mTa 181Ta 182Ta 183Ta
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 177Ta | Syn | – | 56.55 h |
| 178Ta | Syn | – | 2.35 h |
| 179Ta | Syn | – | 1.82 y |
| 180Ta | Syn | – | 8.125 h |
| 180mTa | 0.012 | 179.947 | Stable |
| 181Ta | 99.988 | 180.948 | Stable |
| 182Ta | Syn | – | 114.45 d |
| 183Ta | Syn | – | 5.1 d |
Chemical Reactions of Tantalum
It doesn’t react with air and water under normal condition.
Reacts with halogens upon heating, and forming Tantalum (V) halides.
2 Ta (s) + 5 F2 (g) → TaF5 (s) [white] (Tantalum (V) fluoride)
2 Ta (s) + 5 Cl (g) → TaCl5 (s) [white] (Tantalum (V) chloride)
2 Ta (s) + 5 Br2 (g) → TaBr5 (s) [pale yellow] (Tantalum (V) bromide)
2 Ta (s) + 5 I2 (g) → TaI5 (s) [black] (Tantalum (V) iodide)
Production
Equation of its extraction begins with leaching the Ore with Hydrofluoric acid:
Ta2O5 + 14 HF → 2 H2[TaF7] + 5 H2O
Occur analogous reactions for the niobium component, but for this extraction condition the hexafluoride is typically predominant:
Nb2O5 + 12 HF → 2 H[NbF6] + 5 H2O
H2[TaF7] is treated with potassium fluoride (KF) to produce potassium heptafluorotantalate:
H2[TaF7] + 2 KF → K2[TaF7] + 2 HF
Now, It converted to metallic tantalum by reduction with sodium (at 800 oC) in moltan salt:
K2[TaF7 + 5 Na → Ta + 5 NaF + 2 KF
Tantalum History
Naming: From king Tantalus of Greek mythology.
Discovery: Anders Gustaf Ekeberg (1802) in Uppsala, Sweden
Recognized as a distinct element: Heinrich Rose (1844)
Tantalum Uses
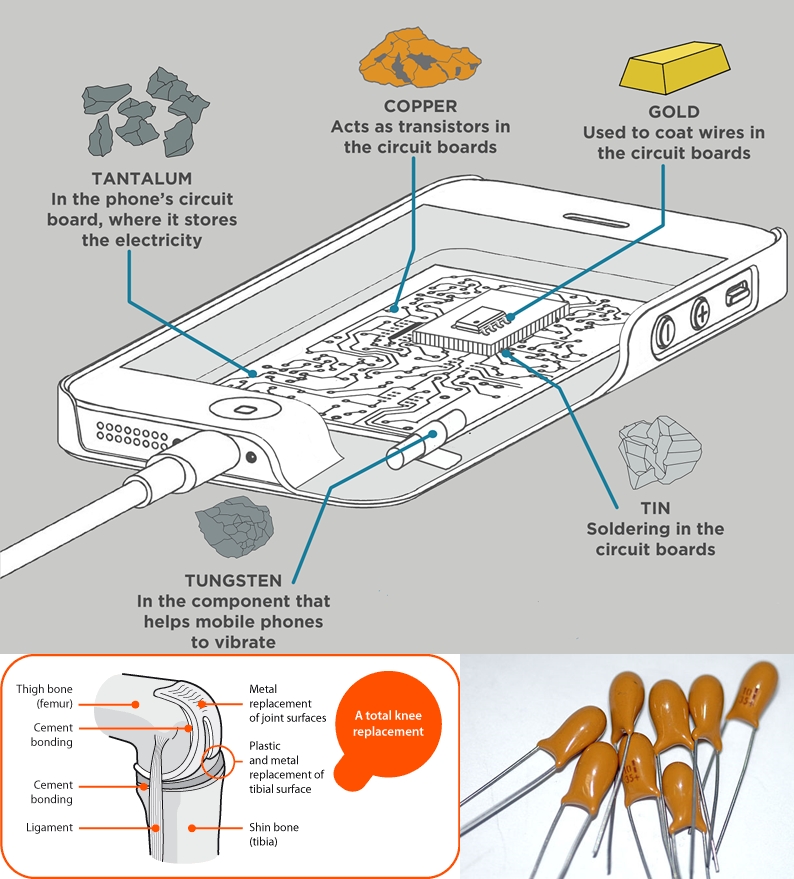
Tantalum is mainly use in the production of electronic components, because the oxide layer which forms on the surface of the metal, that can act as an insulating (dielectric) layer.
It can be used to coat other metals with a very thin layer, to achieve the high capacitance range.
Today, Tantalum capacitors is used in many electronic equipments, such as DVD player, mobile phones, computers etc..
The metal is highly biocompatible (completely immune to body liquids and is a nonirritating material), therefore it is used for body implants.
It has good corrosive resistance property and, therefore it is used in equipment for handling corrosive materials.
It is also impervious (not allowing fluid to pass through) to chemical attack, for this reason it is also uses in chemical industry, as like heat exchanger in boilers where strong acids are vaporized).
Tantalum oxide (TaO) has high index of refraction, so it is used to make special glass for camera lenses.
It has also uses as electrodes for neon lights, AC/DC rectifiers, and many other uses.
Tantalum alloys are extremely strong and have been used for rocket nozzles, turbine blades, missile partsand nose caps for supersonic aircraft.
Tantalum carbide graphite composite material is one of the hardest materials.
Its Compounds has a high melting point (3738 oC), where the metal (60%) is used to make electrolytic capacitors and vaccium furnace parts.
Biological role of Tantalum
It is non-toxic, but The NIOSH & OSHA set a recommended exposure limit of 5 mg/m3 (over an 8 hour workday), and at levels of 2500 mg/m3 (it is immediately dangerous to life & health).
People can be exposed to tantalum by breathing, eye contact, or skin contact.
Abundance of Tantalum
Tantalum is rarely found uncombined in nature, It occurs mainly in the mineral tantalite(Fe,Mn)Ta2O6) & columbite (Fe2+Nb2O6), which also contains other metals including niobium.
There are several complicated steps require to separation of tantalum from niobium .
Several methods are used to produce the metal commercially by electrolysis of molten potassium fluorotantalate (K2[TaF7]), reduction of potassium fluorotantalate with sodium, or reacting tantalum carbide (TaCx) with tantalum oxide(TaO), and it is also produced as a by-product of tin extraction.
Annual world wide production is around 1000 tons.
8×10-9% (In Universe)
2×10-5% (In Meteorites)
0.00017% (In Earth’s Crust)
2×10-10% (In Oceans)


World’s Top 3 producers of Tantalum
1) Brazil
2) Rwanda
3) China
World’s Top 3 Reserve holders of Tantalum
1) Brazil
2) Australia
3) Mozambique
Tantalum Price: Pure (99.9%) Tantalum metal price is around $550-$650 per KG (KiloGram)
#Tantalum