39 Y (Yttrium)

Yttrium is a highly crystalline iron-gray, silver-metallic luster, rare-earth metal.
It is stable in air, because it is proteced by the formation of a stable oxide film on its surface, but oxidizes readily when heated.
It reacts with water and mineral acids.
Shavings or turnings of the metal can ignite in air when they exceed 400 °C
Finely divided yttrium is very unstable in air.
Yttrium nitride is formed when the metal is heated to 1000 oC in nitrogen.

Identity
CAS Number: CAS7440-65-5
CID Number: CID23993
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSZG2980000
CONTENT INDEX
Basic Properties of Yttrium
Pronunciation: it-ree-am
Appearance: Silvery white
Mass Number: 89
Standard Atomic weight: 88.905 g/mol
Atomic number (Z): 39
Electrons: 39
Protons: 39
Neutrons: 50
Period: 5
Group: 3
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M18, N9, O2
Electron configuration: 1s22s22p63s23p63d104s24p64d15s2

Thermal Properties of Yttrium
Phase: Solid
Melting point: 1799 K (1526 oC, 2779 oF)
Boiling point: 3203 K (2930 oC, 5306 oF)
Debye temperature: 280 K (6.85 oC, 44.33 oF)
Fusion heat: 11.42 kJ/mol
Vaporization heat: 363 kJ/mol
Specific heat: 298 J/(kg K)
Molar heat capacity: 26.53 J/(mol.K)
Thermal expansion: α poly: 596 μm/(m∙K)
Thermal conductivity: 17.2 W/(m∙K)
Electrical properties of Yttrium
Electrical conductivity: 1.8×106 S/m
A Electrical resistivity: α poly: 596 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 1.3 K (-271.85 oC, -457.33 oF)
Magnetic Properties of Yttrium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +2.15×10-6 cm3/mol
Volume magnetic susceptibility: 0.0002978
Mass magnetic susceptibility: 66.6×10-9 m3/kg
Molar magnetic susceptibility: 5.921×10-9 m3/mol
Physical Properties of Yttrium
Density: 4.472 g/cm3 (In solid) 4.24 g/cm3 (In Liquid)
Molar volume: 0.000019881 m3/mol
Young’s modulus: 63.5 GPa
Shear modulus: 25.6 GPa
Bulk modulus: 41.2 GPa
Poisson ratio: 0.243
Vicker hardness: 904 MPa
Brinell hardness: 200-589 MPa
Sound Speed: 3300 m/s
Atomic Properties of Yttrium
Oxidation states: 3, 2, 1
Valence Electrons: 4d1 5s2
Ion charge: Y3+
The ionization potential of an atom: 6.5
Ionization energies: 1st: 600 kJ.mol 2nd: 1180 kJ/mol 3rd: 1980 kJ/mol
Ionic radius: 90 pm
Atomic radius: empirical: 180 pm
Van der Waals: 219
Covalent radius: 190±7 pm
Filling Orbital: 4d1
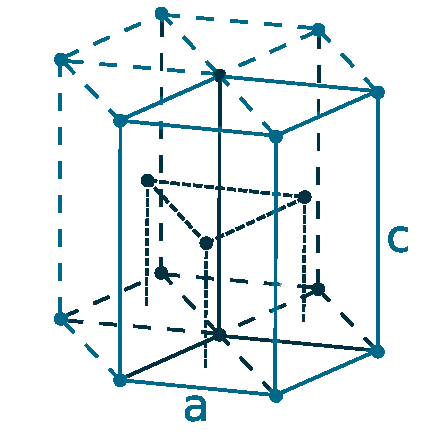
Crystal structure: Hexagonal close-packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 364.7, 364.7, 573.1 pm
Grid parameters: a=3.647 Å c=5.731 Å
Attitude c/a: 1.571
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Yttrium
Electronegativity: pauling scale: 1.22
Valence: +3
Electron affinity: 29.6 kJ/mol
Nuclear Properties of Yttrium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2D3/2
Neutron cross section (Brans): 1.28
Neutron Mass Absorption: 0.00059
Isotopes: 87Y 88Y 89Y 90Y 91Y
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 87Y | Syn | – | 3.4 d |
| 88Y | Syn | – | 106.6 d |
| 89Y | 100 | 88.906 | Stable |
| 90Y | Syn | – | 2.7 d |
| 91Y | Syn | – | 58.5 d |
Chemical Reactions of Yttrium
The metal tarnishes slowly in air and burns readily, forming:
4 Y (s) + 3 O2 (g) → 2 Y2O3 (s) (Yttrium (lll) oxide)
When finely divided, or heated, yttrium metal dissolves in water, forming Yttrium (III) ions and hydrogen gas:
2 Y (s) + 6 H2O (g) → 2 Y3+ (aq) + 6 OH– (aq) + 3 H2 (g)
The metal reacts with all Halogens, forming Yttrium (lll) halides:
2 Y (s) + 3 F2 (g) → 2 YF3 (s) (Yttrium (lll) fluoride)
2 Y (s) + 3 Cl2 (g) → 2 YCl3 (s) (Yttrium (lll) chloride)
2 Y (s) + 3 Br2 (g) → 2 YBr3 (s) (Yttrium (lll) bromide)
2 Y (s) + 3 I2 (g) → 2 YI3 (s) (Yttrium (lll) iodide)
Dissolves readily in dilute hydrochloric acid, forming aquated Yttrium (III) ions and hydrogen gas:
2 Y (s) + 6 HCl → 2 Y3+ (aq) + 6 Cl– (aq) + 3 H2↑ (g)
Yttrium History
Naming: After Ytterby (Sweden) and its mineral ytterbite (gadolinite)
Discovery: Johan Gadolin (1794)
First isolation: Heinrich Rose (1843)
Yttrium Uses:
Yttrium is used as an additive (0.1 to 0.2%) to reduce the grain size in chromium, zirconium, molybdenum, and titanium, and to increases the strength of aluminium and magnesium alloys.
The metal can be used as a deoxidizer for vanadium and other nonferrous metals.
Yttrium can be used as a catalyst for ethylene polymerization reactions.
Yttrium is suitable to make Superconductors.
A Yttrium oxide (yttria) Is the most important compounds of yttrium and It is widely used in making YVO4 europium, and Y2O3 europium phosphors to give the red color in color television tubes.
Yttrium oxide is added to the glass which is used to make camera lenses to make them heat and shock resistant.
Yttrium oxide is also used to produce yttrium-iron-garnets (Y3Fe5O12), which are very effective microwave filters for radar
YIG is also exceptionally efficient as both a transmitter and transducer of acoustic energy.
Yttrium-aluminium garnet (Y3Al5O12) is used in lasers that can cut through metals, It is also used in white LED lights.
YAG with a hardness of 8.5, is also finding use as a gemstone (simulated diamond).
Yttrium Aluminum, Iron, and Gadolinium garnets have interesting magnetic properties.
90Y is the Radioactuve isotope of yttrium, used in medical field (to treat some cancers, such as liver cancer).
Isotope-90Y is exists in equilibrium with its parent 90Sr (isotope of Strontium), a product of nuclear explosions.
Biological role: Its soluble salts are mildly toxic.
Abundance of Yttrium
Yttrium is recovered commercially from ‘rare earth’ minerals monazite sand and bastnasite, which contains about 3% and 0.2% respectively.
Xenotime can contain up to 50% yttrium phosphate.
The metal is produced commercially by reduction of the fluoride with calcium metal.
Annual world wide production is around 8000 tons.
7×10-7% (In Universe)
19×10-5% (In Meteorites)
9.9×10-7% (In Sun)
0.013% (In Earth’s Crust)
1.3×10-9% (In Oceans)

World’s Top 3 producers of Yttrium
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders of Yttrium
1) China
2) CIS Countries (inc. Russia)
3) USA
#Yttrium


