60 Nd (Neodymium)

Neodymium has a bright silvery-yellow metallic luster.
It is very reactive metal and quickly tarnishes in air and the coated formed on the metal does not protect it from further oxidation, So it should be stored in light mineral oil or in sealed in plastic material to prevent from contact with air.
It react slowly with cold water and rapidly with hot water.

Identity
CAS Number: CAS7440-00-8
CID Number: CID23934
RTECS Number: RTECSQ08575000
CONTENT INDEX
Basic Properties of Neodymium
Pronunciation: Nee-oh-dim-ee-am
Appearance: Silvery white
Mass Number: 144
Standard Atomic weight: 144.242 g/mol
Atomic number (Z): 60
Electrons: 60
Protons: 60
Neutrons: 84
Period: 6
Block: f
Element category: Lanthanide
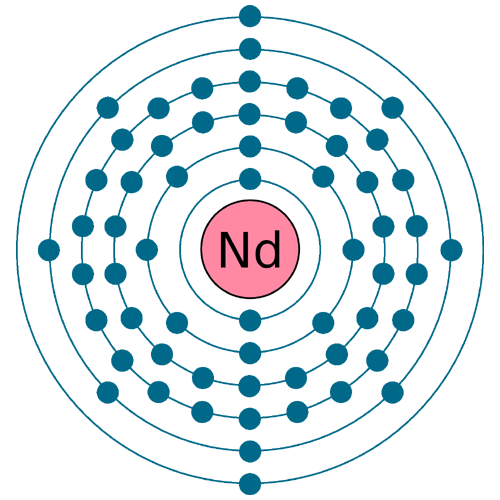
Electrons per shell: K2, L8, M18, N22, O8, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f46s2
Thermal Properties of Neodymium
Phase: Solid
Melting point: 1297 K (1024 oC, 1875 oF)
Boiling point: 3347 K (3074 oC, 5565 oF)
Fusion heat: 7.14 kJ/mol
Vaporization heat: 289 kJ/mol
Molar heat capacity: 27.45 J/(mol.K)
Thermal expansion: α, poly: 9.6 μm/(m∙K)
Thermal conductivity: 16.5 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: 19.2 K (Temperature, above which an antiferromagnetic material becomes paramagnetic)
Electrical properties of Neodymium
Electrical conductivity: 1.6×106 S/m
A Electrical resistivity: α, poly: 640 μΩ∙m
A Electrical type: Conductor
Magnetic Properties of Neodymium
A Magnetic type: Paramagnetic (But Antiferromagnetic below 20 K)
Magnetic susceptibility (xmol): +5628×10-6 cm3/mol
Volume magnetic susceptibility: 0.0033648
Mass magnetic susceptibility: 480×10-9 m3/kg
Molar magnetic susceptibility: 640×10-9 m3/mol
Physical Properties of Neodymium
Density: 7.01 g/cm3 (In solid) 6.89 g/cm3 (In Liquid)
Molar volume: 0.00002058 m3/mol
Young’s modulus: α form: 41.4 GPa
Shear modulus: α form: 16.3 GPa
Mohs Hardness: 1.23
Bulk modulus: α form: 31.8 GPa
Poisson ratio: α form: 0.281
Vicker hardness: 345-745 MPa
Brinell hardness: 265-700 MPa
Sound Speed: 2330 m/s
Atomic Properties of Neodymium
Oxidation states: +4,+3,+2
Valence Electrons: 4f4 6s2
Ion charge: Nd3+
Ionization energies: 1st: 533.1 kJ.mol 2nd: 1040 kJ/mol 3rd: 2130 kJ/mol
Ionic radius: 99.5 pm
Atomic radius: 229 pm (Van der Waals)
Covalent radius: 201±6 pm
Filling Orbital: 4f4
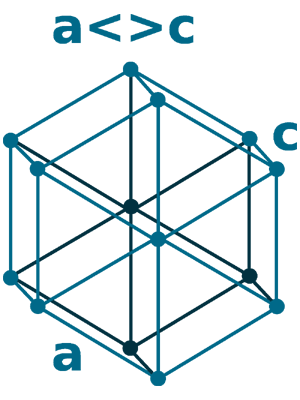
Crystal structure: Double hexagonal close packed (body-centered cubic structure taking place at 863°C)
Grid parameters: a=3.658 Å c=11.80 Å
Attitude c/a: 3.23
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Neodymium
Electronegativity: pauling scale: 1.14
Valence: +3
Electron affinity: 50 kJ/mol
Nuclear Properties of Neodymium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 5I4
Neutron cross section (Brans): 49
Neutron Mass Absorption: 0.011
Isotopes: 142Nd 143Nd 144Nd 145Nd 146Nd 148Nd 150Nd
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 142Nd | 27.2 | 141.908 | Stable |
| 143Nd | 12.2 | 142.910 | Stable |
| 144Nd | 23.8 | 143.910 | 2.29×1015 y |
| 145Nd | 8.3 | 144.913 | Stable |
| 146Nd | 17.2 | 145.913 | Stable |
| 148Nd | 5.8 | 147.917 | Stable |
| 150Nd | 5.6 | 149.921 | 6.7×1018 y |
Chemical Reactions
Neodymium Burn readily at 150 oC to form Neodymium (lll) oxide:
4 Nd + 3 O2 → 2 Nd2O3
Reacts slowly in cold water and rapidly in hot water (Produce Neodymium (lll) hydroxide):
2 Nd (s) + 6 H2O (l) → 2 Nd(OH)3 (aq) + 3 H2 (g)
The metal reacts with Halogens:
2 Nd (s) + 3 F2 (g) → 2 NdF3 (s) [violet]
2 Nd (s) + 3 Cl2 (g) → 2 NdCl3 (s) [mauve ]
2 Nd (s) + 3 Br2 (g) → 2 NdBr3 (s) [violet]
2 Nd (s) + 3 I2 (g) → 2 NdI3 (s) [green]
Dissolves readily in dilute sulfuric acid:
2 Nd (s) + 3 H2SO4 (aq) → 2 Nd3+ (aq) + 3 SO42−(aq) + 3 H2 (g)
Neodymium History
Discovery: Carl Auer von Welsbach (1885)
Neodymium Uses
Neodymium oxide and nitrate are used as catalysts in polymerisation reactions.
A Neodymium magnets, which alloyed with Iron and Boron (Nd2Fe14B alloy) makes very strongest permanent magnets.
A Neodymium magnets is widely used in many electronic devices like microphones, loudspeakers, computer hard disks and electronic musical instruments.
This magnets is also used in high performance DC electric motors and electric generators, car windscreen wiper and wind turbines.
Didymium (a mixture of Neodymium and Praseodymium) is used for coloring glass to make welders goggles, which is able to absorb the yellow sodium glare of the flame. This kind of glass is used to protect the eyes of welders.
Neodymium colors glass delicate shades of violet, wine-red and warm grey.
The glass also used in astronomical work to produce sharp bands by which spectral lines may be calibrated.
Neodymium Glass can be used as laser pointers to produce coherent light, as well as in eye surgery, cosmetic surgery and for the treatment of skin cancers.
Biological role: It is moderately toxic and irritating to eyes. It should be handle with care.
Abundance of Neodymium
Neodymium is Found in the minerals monazite and bastnaesite.
It commercially extracted by ion exchange and solvent extraction techniques.
It can also be obtained by reducing anhydrous halides such as neodymium chloride (Ndcl3) or fluoride (Ndf3) with calcium.
Annual world wide production is around 7300 tons.
1×10-6% (In Universe)
5×10-5% (In Meteorites)
3×10-7% (In Sun)
0.0033% (In Earth’s Crust)
2.8×10-10% (In Oceans)
World’s Top 3 producers of Neodymium
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders of Neodymium
1) China
2) CIS Countries (inc. Russia)
3) USA
#neodymium


