78 Pt (Platinum)

Pure Platinum is a malleable, ductile, lustrous, silvery-white metal.
It has the 3rd highest density, after osmium and iridium.
The metal unaffected by air & water, and doesn’t oxidize at any temperature, but it is corroded by halogens, Sulfur, cyanides, and caustic alkalis.
It is insoluble in hydrochloric (HCl) and nitric acid (HNO3), but dissolves in aqua regia (a mixture of concentrated nitric and hydrochloric acids), and forming chloroplatinic acid.
It also dissolves in hot concentrated phosphoric (H3PO4) & sulphuric acids (H2SO4), and in molten alkali.


Identity
CAS Number: CAS7440-06-4
CID Number: CID23939
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSTP2160000
CONTENT INDEX
Basic Properties of Platinum
Pronunciation: Plat-a-nam
Appearance: Silvery white
Mass Number: 195
Standard Atomic weight: 195.084 g/mol
Atomic number (Z): 78
Electrons: 78
Protons: 78
Neutrons: 117
Period: 6
Group: 10
Block: d
Element category: Transition metal
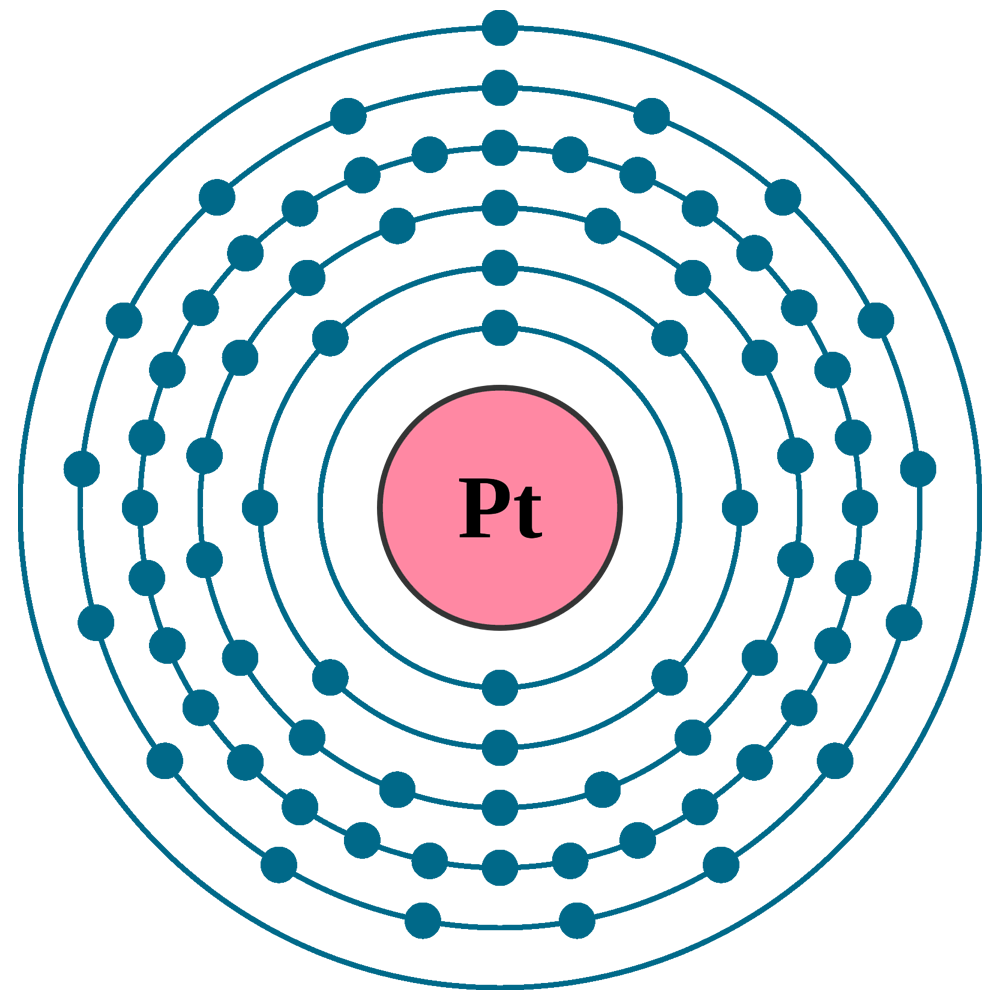
Electrons per shell: K2, L8, M18, N32, O17, P1
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f145d96s1
Thermal Properties of Platinum
Phase: Solid
Melting point: 2041.4 K (1768.3 oC, 3214.9 oF)
Boiling point: 4098 K (3825 oC, 6917 oF)
Debye temperature: 230 K (-43.15 oC, -45.67 oF)
Fusion heat: 22.17 kJ/mol
Vaporization heat: 510 kJ/mol
Specific heat: 133 J/(kg K)
Molar heat capacity: 25.86 J/(mol.K)
Thermal expansion: 8.8 μm/(m∙K)
Thermal conductivity: 71.6 W/(m∙K)
Electrical properties of Platinum
Electrical conductivity: 9.4×106 S/m
A Electrical resistivity: 105 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Platinum
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +202×10-6 cm3/mol
Volume magnetic susceptibility: 0.0002573
Mass magnetic susceptibility: 12.2×10-9 m3/kg
Molar magnetic susceptibility: 2.38×10-9 m3/mol
Physical Properties of Platinum
Density: 21.45 g/cm3 (In solid) 19.77 g/cm3 (In Liquid at M.P)
Molar volume: 0.000009094 m3/mol
Tensile strength: 125-240 MPa
Young’s modulus: 168 GPa
Shear modulus: 61 GPa
Mohs Hardness: 3.5
Bulk modulus: 230 GPa
Poisson ratio: 0.38
Vicker hardness: 400-550 MPa
Brinell hardness: 300-500 MPa
Sound Speed: 2800 m/s
Atomic Properties of Platinum
Oxidation states: -3, -2, -1, 1, 2, 3, 4, 5, 6
Valence Electrons: 5d9 6s1
Ion charge: Pt4+ Pt2+
Ionization potential of an atom: 8.9
Ionization energies: 1st: 870 kJ.mol 2nd: 1790 kJ/mol
Ionic radius: 62.5 pm
Atomic radius: 139 pm (empirical)
Van der Waals: 175 Pm
Covalent radius: 136±5 pm
Filling Orbital: 5d9
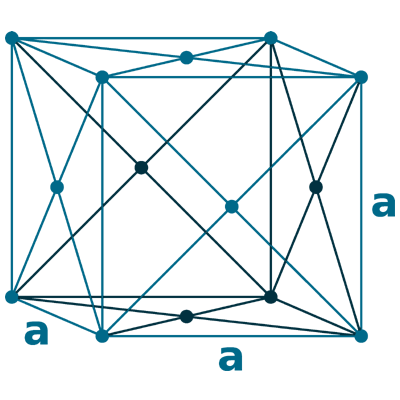
Crystal structure: Face centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 392.1, 392.1, 392.1 pm
Grid parameters: a=3.920 Å
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Platinum
Electronegativity: 2.28 (pauling scale)
Valence: +6
Electron affinity: 205 kJ/mol
Nuclear Properties of Platinum
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3D3
Neutron cross section (Brans): 10
Neutron Mass Absorption: 0.002
Isotopes: 190Pt 192Pt 193Pt 194Pt 195Pt 196Pt 198Pt
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 190Pt | 0.012 | 189.961 | 6.5×1011 y |
| 192Pt | 0.782 | 191.963 | Stable |
| 193Pt | Syn | – | 50 y |
| 194pt | 32.864 | 193.965 | Stable |
| 195Pt | 33.775 | 194.965 | Stable |
| 196Pt | 25.211 | 195.966 | Stable |
| 198Pt | 7.356 | 197.969 | Stable |
Chemical Reactions of Platinum
The metal reacts with Halogens, and forming platinum halides, depending on reaction conditions.
Reacts with fluorine, and forms:
Pt (s) + 3 F2 (g) → PtF6 (s) [dark red] (platinum (VI) fluoride)
4 Pt (s) + 10 F2 (g) → (PtF5)4 (s) [deep red] (platinum (V) fluoride)
In this reaction, Tetrameric platinum(V) fluoride disproportionates into:
(PtF5)4 (s) → PtF6 (s) + PtF4 (s) [yellow brown]
It Reacts with Chlorine, and forms:
Pt (s) + Cl2 (g) → PtCl2 (s) [brown black] (Platinum (II) chloride)
Pt (s) + 2 Cl2 (g) → PtCl4 (s) [dark red or olive green] (Platinum(IV) chloride)
Reacts with bromine & iodine, and forms:
Pt (s) + 2 Br2 (g) → PtBr4 (s) [brown black] (Platinum (IV) chloride)
Pt (s) + 2 I2 (g) → PtI4 (s) [brown black] (platinum (IV) iodide)
Platinum History
Naming: Spanish: platina (little silver).
Discovery: Antonio de Ulloa (1735) in South America
Platinum Uses
Almost 35% of Platinum is extensively used in jewelry.
It is most common used as a catalyst in form of platinum black for chemical reactions in catalytic converter for truck, car, and buses, where it is very effective to coverting emission into less harmful waste products.
As a catalyst, it is also used in industries for the production of Silicone, Nitric acid, and benzene, where it improves the efficiency of fuel cells.
Almost 45% of aplatinum are used in field of vehicle emission control devices, and 10% are used in chemical production & petroleum refining, and its demand is increasing every year.
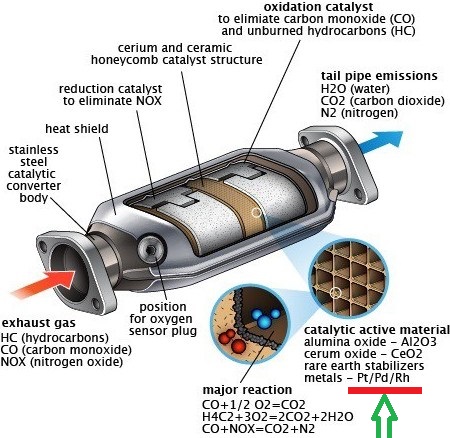
Other minor uses of platinum are in glassmaking equipment, electrodes, corrosion-resistant apparatus, electrical contacts, oxygen sensors, medicine, anticancer drugs, spark plugs, turbine engines, vessels for laboratory use, pacemakers, dental fillings, and in many valuable instruments including thermocouple elements.
The electronics industry uses platinums to make optical fibres, LCDs, in Computer hard drives etc…
Platinum-cobalt alloys (76.7% Pt, 23.3% Co) have magnetic properties, which is an extremely powerful magnet.
It is used in electrical resistance wires for constructing high-temperature electric furnaces.
The metal is used for coating missile nose cones, turbine blades, jet engine fuel nozzles, spark plugs etc., which must perform reliably at high temperatures for long periods of time.
APlatinum-based antineoplastic (antitumor) agents are important chemotherapy drugs, which is used to treat cancers, and shows good activity against some tumors.
Biological role of Platinum
It is non-toxic & not very dangerous, but some of its salts can cause several health effects, Such as irritation of the eyes, nose, throat are short-term exposure, and respiratory & skin allergies are long-term exposure.
Some Health organizations has set a recommended exposure limit (REL) for platinum is 1 mg/m3 over an 8-hour workday.
Abundance of Platinum
APlatinum is found uncombined in alluvial deposits (Material deposited by rivers) of the Ural mountains, of Columbia, and of certain western American states.
Commercially, Platinum is produced from mineral Cooperite (platinum sulfide, PtS), which is mostly found in south africa.
Some platinum is prepared as a by-product of nickel & copper refining.
Sperrylite (Platinum arsenide, PtAs2) is a major source of platinum, which is occurring with the nickel-bearing deposits of Sudbury (Ontario).
Annual world wide production of Platinum is around 250 tons.
5×10-7% (In Universe)
9.8×10-5% (In Meteorites)
9×10-7% (In Sun)
3.7×10-6 % (In Earth’s Crust)


World’s Top 3 producers of Platinum
1) South Africa
2) Russia
3) Zimbabwe
World’s Top 3 Reserve holders of Platinum
1) South Africa
2) Russia
3) USA
Platinum Price
Pure (99.99%) metal price is around $27,000-$31,000 per KG (KiloGram)
#Platinum


