05 B (Boron Element)

CONTENT INDEX
About Boron Element
Boron is a non-metallic element, and it is the only non-metal of the group-13 of the periodic table elements. It is a poor conductor of electricity at normal or Room temperature, but a good conductor at high temperature.


The most common form (Pure form) of Boron is Amorphous boron (A dark powder), which is unreactive to Oxygen, Water, Acids, and Alkalis. But it reacts with metals and forms borides.
Identity
CAS Number: CAS7440-42-8
CID Number: CID5462311
DOT Hazard Class: N/A
DOT Number: N/A
RTECS Number: RTECSED7350000
Properties of Boron
Basic Properties of Boron
Pronunciation: (Bohr-on / bo-ron)
Allotropes: α-rhombohedral, β-rhombohedral, β-tetragonal and more
Appearance: Black-brown
Mass Number: 10
Standard Atomic weight: 10.81 g/mol
Atomic number (Z): 5
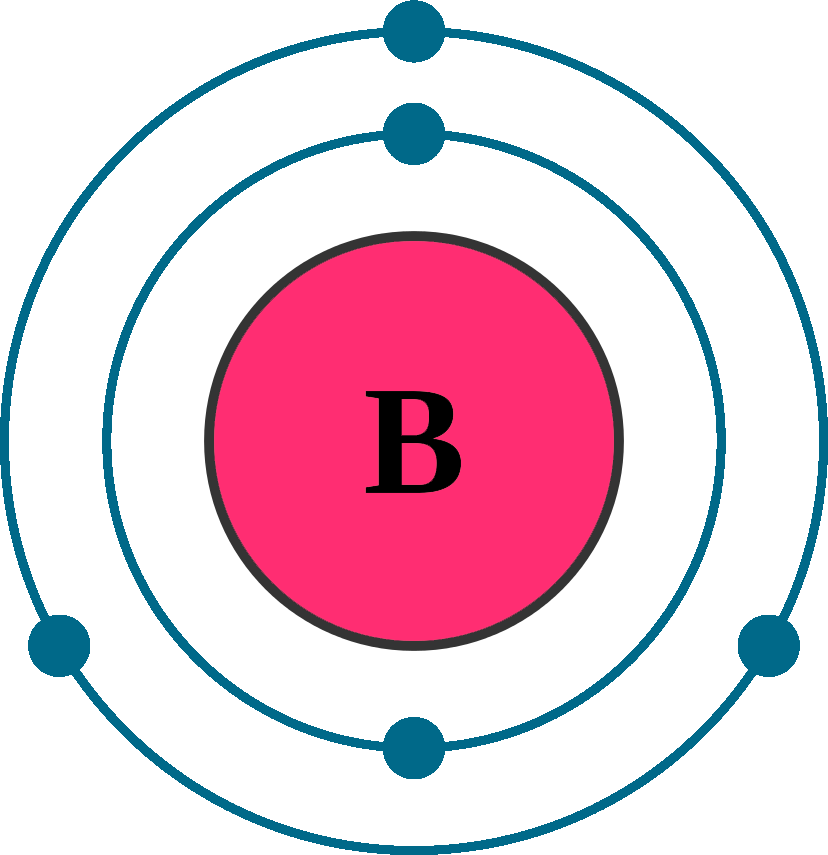
Electrons: 5
Protons: 5
Neutrons: 6
Period: 2
Group: 13
Block: p
Element category: Metalloid
Electrons per shell: K2, L3
Electron configuration: 1s22s22p1
Thermal Properties of Boron
Phase: Solid
Melting point: 2348 K (2075 oC, 3767 oF)
Boiling point: 4200 K (3927 oC, 7100 oF)
Debye temperature: 1250 K (976.85 oC, 1790.33 oF)
Fusion heat: 50.2 kJ/mol
Vaporization heat: 508 kJ/mol
Specific heat: 1030 J/(kg K)
Molar heat capacity: 11.08 J/(mol.K)
Thermal expansion: β: 5-7 μm/(m∙K)
Thermal conductivity: 27.4 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: N/A
Electrical properties of Boron
Electrical conductivity: 0.0001 S/m
Electrical resistivity: -106 μΩ∙m
Electrical type: Dielectric
Resistivity: 10000 mΩ
Critical point (Superconducting point): N/A
Magnetic Properties of Boron
Magnetic type: Diamagnetic
Curie point: N/A
Magnetic susceptibility (xmol): -6.7×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000214
Mass magnetic susceptibility: -8.7×10-9 m3/kg
Molar magnetic susceptibility: -0.0941×10-9 m3/mol
Physical Properties of Boron
Density: 2.08 g/cm3 (In liquid)
Molar volume: 0.00002045 m3/mol
Young’s modulus: N/A
Shear modulus: N/A
Mohs Hardness: 9.3
Bulk modulus: 320 GPa
Poisson ratio: N/A
Vicker hardness: 49000 MPa
Brinell hardness: N/A
Sound Speed: 16,200 m/s
Atomic Properties of Boron
Oxidation states: -5, -1, +1, +2, +3
Valence Electrons: 2s2 2p1
Ion charge: N/A
The ionization potential of an atom: 8.33 eV
Ionization energies: 1st: 800.5 kJ.mol 2nd: 2427 kJ/mol 3rd: 3659.5 kJ/mol
Ionic radius: 23 pm
Atomic radius: 90 pm (empirical)
Van der Waals: 192 Pm
Covalent radius: 84±3 pm
Filling Orbital: 2p1
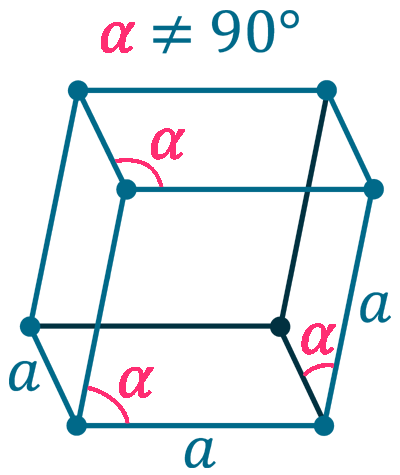
Crystal structure: Rhombohedral
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 1017, 1017, 1017 pm
Grid parameters: a=10.17 Å, α=65.18 Å
Attitude c/a: 0.576
Space Group Name: R_3m
Space Group Number: 166
Reactivity of Boron
Electronegativity: 2.04 (pauling scale)
Valence: 3
Electron affinity: 26.7 kJ/mol
Nuclear Properties of Boron
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2P1/2
Neutron cross section (Brans): 760
Neutron Mass Absorption: 2.4
Isotopes: 10B 11B
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 10B | 20 | 10.012 | Stable |
| 11B | 80 | 11.008 | Stable |
Chemical Reactions of Boron
Reaction with Air
Boron doesn’t react with air at room temperature. But at high temperature, it reacts with Oxygen and forming boron (III) oxide (B2O3).
4 B (s) + 3 O2 (g) → 2 B2O3 (s)
Reaction with Water
It doesn’t react with water under normal condition.
Reaction with Halogens
Boron reacts with Fluorine, Chlorine, Bromine and forming the Boron (III) trihalides.
2 B (s) + 3 F2 (g) → 2 BF3 (g) (Boron (lll) fluoride)
2 B (s) + 3 Cl2 (g) → 2 BCl3 (l) (Boron (lll) chloride)
2 B (s) + 3 Br2 (g) → 2 BBr3 (l) (Boron (lll) bromide)
Reaction with Acids
Crystalline boron doesn’t react with Boiling HydroFluoric acid (HF) or HydroChloric acid (HCL). But Amorphous (Powdered) boron oxidizes slowly when treated with concentrated Nitric Acid (HNO3).
History of Boron Element
Naming Origin: From borax and carbon.
Discovery: Joseph Louis Gay-Lussac and Louis Jacques Thénard (30 June 1808)
First isolation: Humphry Davy (9 July 1808)
Boron Uses
Amorphous (Non crystalline) boron is used in pyrotechnic flares to provide a distinctive green color, and in Rockets as a Fuel igniter. The most commercially important boron compound is Borax (sodium tetraborate) Pentahydrate (Na2B4O7.5H2O), which is used to a large scale in the manufacture of insulation fibreglass and sodium perborate bleach.

Boric (or boracic) acid (H3BO3), Borax (sodium borate) and Boric oxide (The pure form of B2O3) are also most important compound of Boron.
Boric acid has antiseptic, antifungal, and antiviral properties, so it is applied as a water clarifier in swimming pool water treatment. Mild solution used as Eye Antiseptics. As well as it is used in Textile Fiberglass, flat panel displays and in many PVAc- and PVOH-based adhesives.
It is also used as an insecticide (against Ants, Fleas, and Cockroaches).
Borax is used in various household laundry and cleaning products like detergents, bleaches etc… and used as a food preservative. It is also used in some anti-corrosion systems, as well as used as a flux for soldering gold and silver and with ammonium chloride for welding ferrous metals.
Sodium octaborate is a flame retardant (prevent fires from starting and spreading).
Boric oxide is extensively used in the manufacture of borosilicate glass (Pyrex) to makes the glass tough and heat resistant. Fibreglass textiles and insulation are made from borosilcate glass.
Other boron compounds show promise in Treating Arthritis.
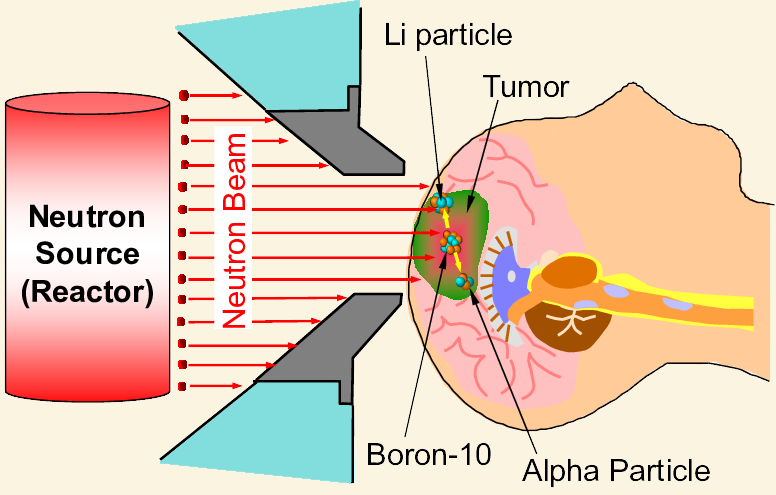
The isotope boron-10 is good for absorbing neutrons, so it is used as a shield to control nuclear radiation in nuclear reactors. B-10 is also used in instruments used for detecting neutrons, as well as used in Boron neutron capture therapy (BNCT).
Boron nitride (BN) is thermally and chemically resistant refractory compound and has a property to make a material hard as diamond.
The Nitride (N3-) also behaves like an Electrical Insulator but conducts heat like a metal.
Boron also has lubricating properties similar to graphite.
Boron filaments (Boron Fiber) are used for advanced aerospace structures, due to their High-strength, Light weight, and High elastic modulus.
Boron is similar to carbon, and it has the capacity to form stable covalently bonded molecular networks.
Metalloboranes, Phosphacarboranes, Carbonates, and other families comprise thousands of compounds.
Biological role of Boron Element
Boron Element and the borates are considered to be non-toxic. However, some of the more exotic boron hydrogen compounds are definitely toxic and require handling with care.
Boron is essential for the cell walls of plants. Plants absorb boron from the ground and through plant-consuming animals it can end up in food chains.
It is not considered poisonous to animals or humans, but in higher doses it can upset the body’s metabolism.
Humans can be exposed to boron through Water, Air, Fruits & Vegetables, and consumer Products (Cometics, Laundry etc…). We intake about 2 milligrams (mg) of boron each day, and about 60 grams in a lifetime.
If we consume a large amount of boron-containing food, it may raise the level of boron that can cause health problems. It can infect the Stomach, Kidneys, Liver, and Brain, which can eventually lead to death.
It can also affect the male reproductive organs and exposed to boron during pregnancy, their child may suffer from birth defects or delayed development.
With exposure to small amounts of boron, irritation in the Throat, Nose, or eyes may occur. 5 gm of boric acid can make us ill and 20 grams or more put our life in danger.
Some boron compounds are being studied as a possible treatment for brain tumors by Boron Neutron Capture Therapy (BNCT).
Boron Sources
Elemental Boron is not found free in nature, It occurs as Orthoboric Acid in some volcanic spring water, and as borates (BO3-3) in Borax (Na2[B4O5(OH)4]·8H2O) and Colemantie (Ca2B6O11·5H2O). Extensive borax deposits are also found in Turkey.


The most important source of boron is Rasorite (kernite, Na2B4O6(OH)2·3H2O) and Tincal (borax ore, Na2B4O7.10H2O), which are found in the Mojave Desert in California (USA).

High-purity crystalline boron is prepared by the vapour phase reduction of boron Trichloride (BCl3) or Tribromide (BBr3) with hydrogen, on electrically heated filaments. Impure (amorphous) boron can be prepared by heating the trioxide (O3) with magnesium powder.
Boron occurs in the environment mainly through natural processes.
It occurs naturally in the environment due to the release into air, soil and water through weathering.
It may also occur in groundwater in very small amounts.
Boron element with purity of 99.9999%, has been produced and is available commercially. Elemental boron has an energy band gap of 1.50 to 1.56 eV, which is higher than that of either Silicon or Germanium.
Annual world wide production of Boron oxide (B2O3) is around 20,00,000 tonnes.
1×10-7% (In Universe)
0.00016% (In Meteorites)
2×10-7% (In Sun)
0.00086% (In Earth’s Crust)
0.00044% (In Oceans)
0.00007% (In Humans)
World’s Top 3 producers of Boron
1) Turkey
2) USA
3) Chile
World’s Top 3 Reserve holders of Boron
1) Turkey
2) Russia
3) USA
Boron Price
Pure (99.95%) Element (Crystalline Boron) price is around $10 per G (Gram)
You Can Buy Pure Boron Element and Boron Products
Element Database
Atomic Spectroscopic Data
→ ASD Line
→ ASD Levels
→ Ground States and Ionization Energies
→ Handbook of Basic ASD
Atomic and Molecular Data
→ Electron-Impact Cross Sections
Bibliographic Databases on Atomic Spectroscopy
→ Atomic Transition Probability Bibliographic Database
→ Atomic Spectral Line Broadening Bibliographic Database
→ Atomic Energy Levels and Spectra Bibliographic Database
X-Ray and Gamma Ray Data
→ X-ray Attenuation and Absorption for Materials of Dosimetric Interest
→ XCOM: Photon Cross Section Database
→ Form Factor, Attenuation, and Scattering Tabulation
Radiation Dosimetry Data
Nuclear Physics Data
Condensed Matter Physics Data
→ Atomic Reference Data for Electronic Structure Calculations
References
Wikipedia
Los Alamos National Laboratory
National Institute of Standards and Technology
Environmental Chemistry
Royal Society of Chemistry
Periodic Table
Lenntech
Web Elements
Michael Pilgaard’s Elements



