To know about mass number, Firstly we should know about atomic mass, atoms and molecules. What is atomic mass?
Atomic weight or Atomic mass
- Mass of an atom is called as atomic mass. Total 118 elements are present in periodic table. But It’s very difficult to find out atomic mass of each atom.
- The atomic mass of an element is the average mass of the atom of that element, it is measured by atomic mass unit which is amu.
- So its unit is amu or u.
- In other words, we can say, it is the average atomic weight of an element, measured in units of atomic mass, also called Dalton[D].
- The atomic weight is the average of the weight of all isotopes of an element, and the mass of each isotope multiplied by the frequency is a specific isotope.
- Atomic mass is also called atomic weight, but the term “mass” is more accurate.
- Carbon-12 has an atomic mass of 1 to 12 times of 1.992646547 x 10-23 gram, and has an atomic mass of 12 units.
- On this scale, one unit of atomic mass (amu) is equivalent to 1.660539040 X 10-24 g.
- As you know that all matters are made up of atoms and molecules in this universe.
- Although all molecules are made up of atoms that’s why atoms are fundamental unit of matters.
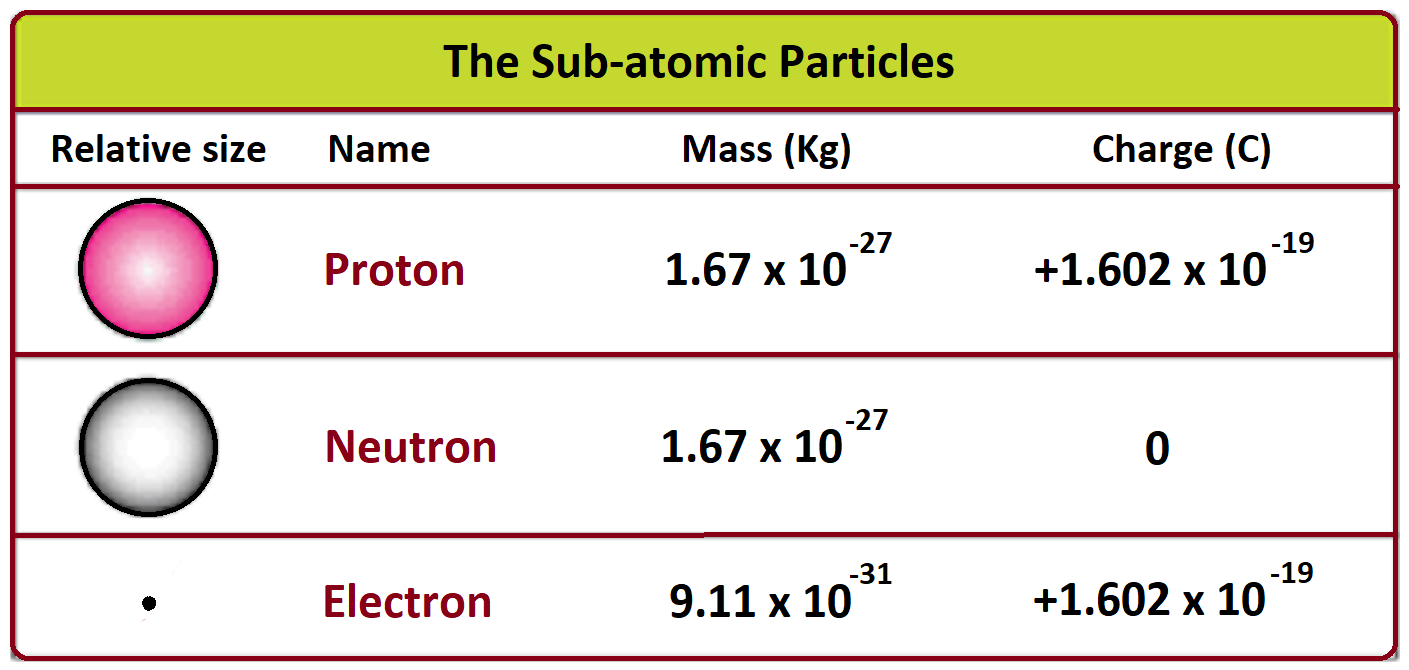
- Atom word is coming from Atomos which means indivisible. But later on, it found that atoms are not indivisible. It contains proton, neutron and electron.
- Generally, protons are positive in charge, electrons are negative, and neutrons are neutral.
Mass number
The mass number of a component is the amount of the quantity of protons and neutrons in that component’s particles. That is,
The mass number of a component = number of protons and number of neutrons.
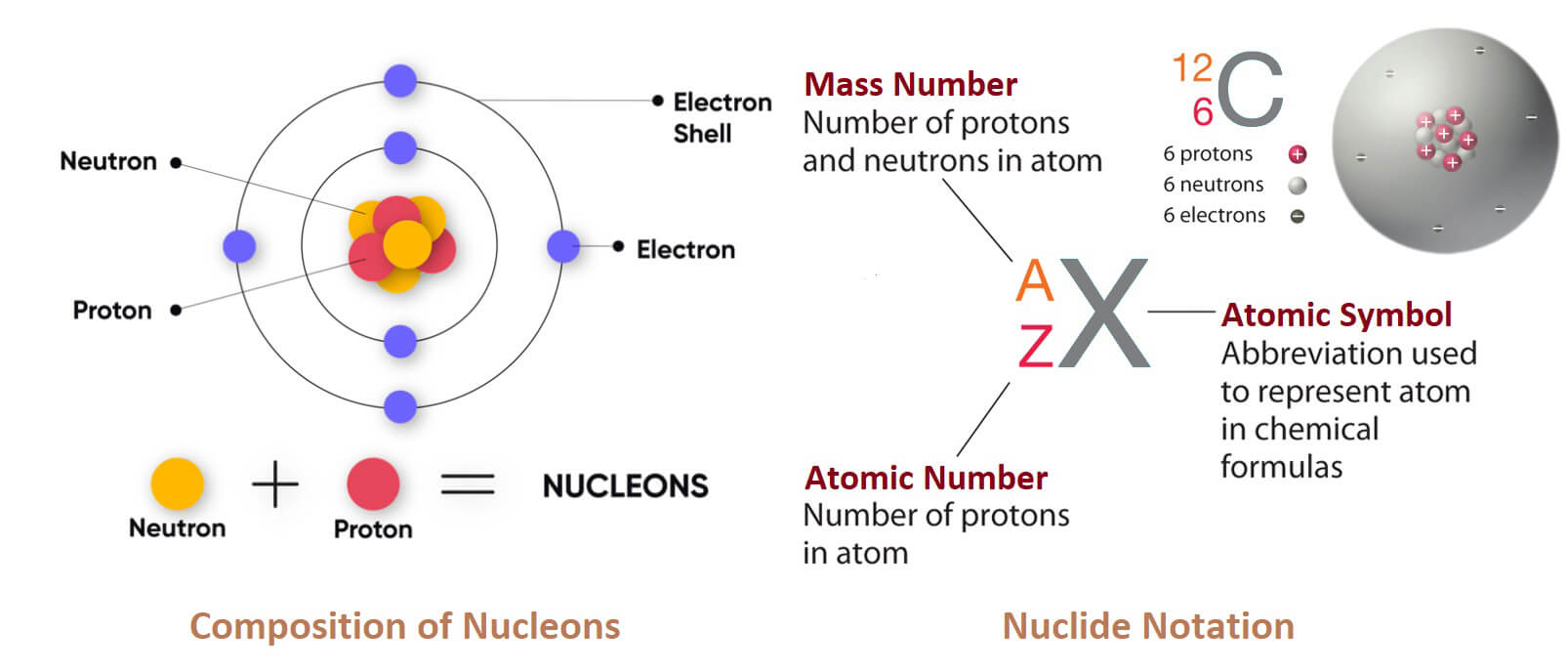
These particles are called nucleons, due to the presence of protons and neutrons in the nucleus.
Representation of atomic number and mass number with the symbol of the element
Atomic number of an element is usually denoted by Z and mass number [atomic mass] is represented by A. They are represented along with the symbol of the element [X] as follows:
How to calculate mass number :
mass number[A]= P+n , P=proton, n=neutron
[A]= Z+n , Z=atomic number, n= number of neutron
n=A-Z
Mass number[A]=Z+n
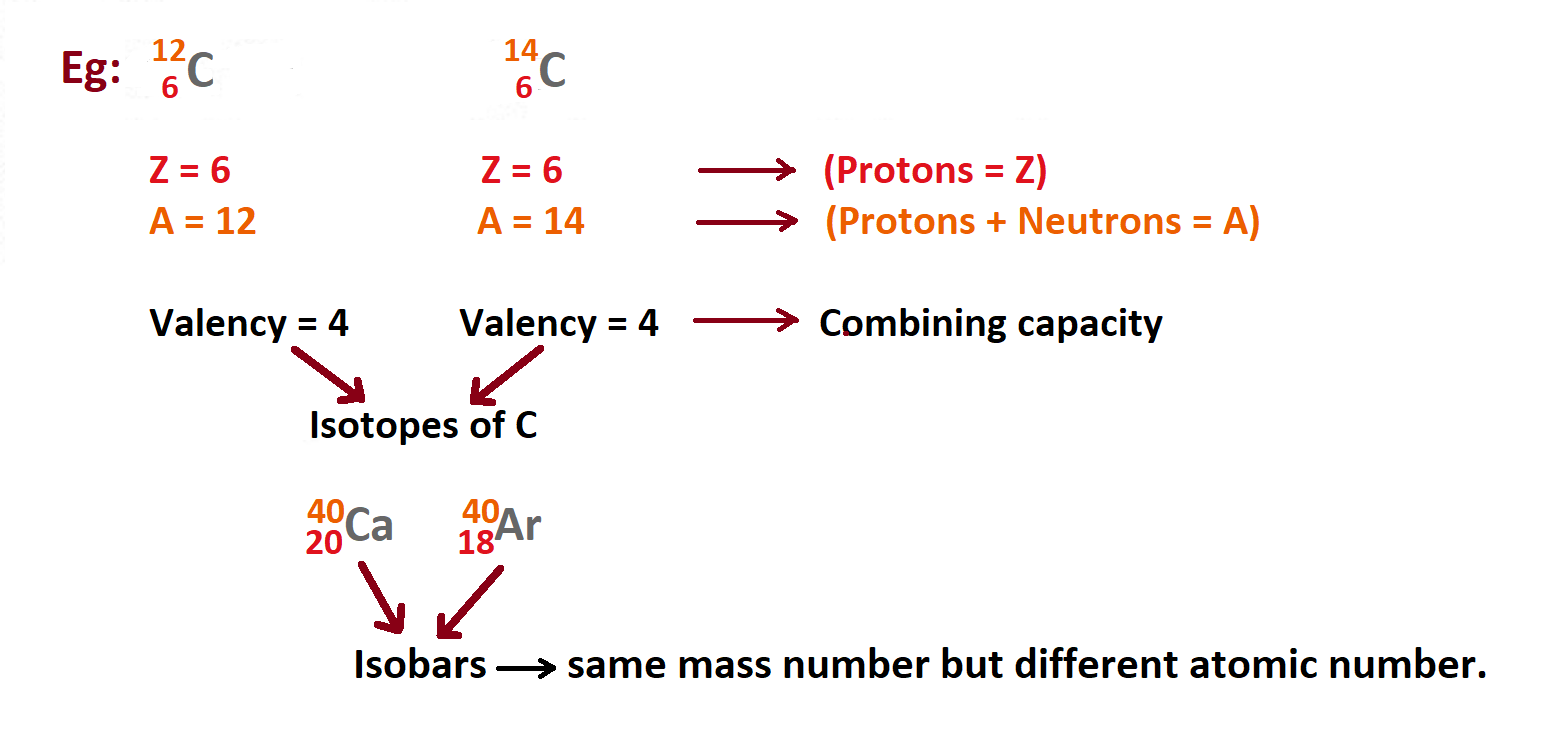
Valency of mass number
The mass of an atom can be calculated by adding the total number of protons and neutrons and multiplying by one atom. A measure of the ability or ability of an element to combine with other atoms to form a compound or molecule. This combining capacity of entity is known as valency.
Mass number of beta particle
Beta particles, also known as beta rays or beta rays (symbols), are fast, energetic positrons or positron electrons that are emitted during the radioactive decay of a nucleus during beta decay.
- There are two kinds of beta decay: beta+ and beta -, which produce positrons and electrons individually.
- Beta particles with an energy of 0.5 MeV are found in air at a range of about 1 m. The distance depends on the energy of the particle.
- Beta particles are a type of ionizing radiation and are considered more ionizing than gamma rays for radiation safety reasons, but less ionizing than alpha particles.
- The greater the ionizing effect, the greater the damage to living tissue, but the less radiation delivered.
- A beta particle has a relative mass of zero, so its mass number is zero.
- Beta particles are electrons, so we can write 0-1e. However, it is sometimes written as 0-1β.
- Beta particles are electrons, but they come from the nucleus, not the atom. So their mass will be (9.109 383 56 ± 0.000 000 11) × 10⁻³¹ kg.