62 Sm (Samarium)

Samarium is a Bright silvery-white metallic lusture, Lanthanide group of the periodic table.
It is relatively stable in dry air at room temperature, but it ignites in air when heated above 150 oC and forms an oxide coating in moist air.
Samarium sulfide(Sm2S3) has excellent high-temperature stability and good thermoelectric efficiencies up to 1100°C.

Identity
CAS Number: CAS7440-19-9
CID Number: CID23951
DOT Hazard Class: 4.1
DOT Number: 1325
CONTENT INDEX
Basic Properties of Samarium
Pronunciation: Sa-mair-ee-am
Appearance: Silvery white
Mass Number: 150
Standard Atomic weight: 150.36 g/mol
Atomic number (Z): 62
Electrons: 62
Protons: 62
Neutrons: 88
Period: 6
Block: f
Element category: Lanthanide
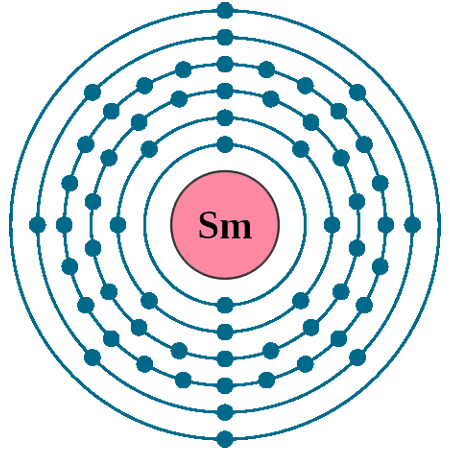
Electrons per shell: K2, L8, M18, N24, O8, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f66s2
Thermal Properties of Samarium
Phase: Solid
Melting point: 1345 K (1072 oC, 1962 oF)
Boiling point: 2173 K (1900 oC, 3452 oF)
Debye temperature: 166 K (-107.15 oC, -160.87 oF)
Fusion heat: 8.62 kJ/mol
Vaporization heat: 192 kJ/mol
Molar heat capacity: 29.54 J/(mol.K)
Thermal expansion: α, poly: 12.7 μm/(m∙K)
Thermal conductivity: 13.3 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: 106 K (Temperature, above which an antiferromagnetic material becomes paramagnetic)
Electrical properties of Samarium
Electrical conductivity: 1.1×106 S/m
a Electrical resistivity: α, poly: 0.940 μΩ∙m
a Electrical type: Conductor
Magnetic Properties of Samarium
a Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +1860×10-6 cm3/mol
Volume magnetic susceptibility: 0.00081618
Mass magnetic susceptibility: 111×10-9 m3/kg
Molar magnetic susceptibility: 16.69×10-9 m3/mol
Physical Properties of Samarium
Density: 7.52 g/cm3 (In solid) 7.16 g/cm3 (In Liquid)
Molar volume: 0.00002045 m3/mol
Young’s modulus: α form: 49.7 GPa
Shear modulus: α form: 19.5 GPa
Mohs Hardness: 1.44
Bulk modulus: α form: 37.8 GPa
Poisson ratio: α form: 0.274
Vicker hardness: 410-440 MPa
Brinell hardness: 440-600 MPa
Sound Speed: 2130 m/s
Atomic Properties of Samarium
Oxidation states: 3,2,
Valence Electrons: 4f6 6s2
Ion charge: Sm3+ Sm2+
Ionization energies: 1st: 544.5 kJ.mol 2nd: 1070 kJ/mol 3rd: 2260 kJ/mol
Ionic radius: 96.4 pm
Atomic radius: 229 pm (Van der Waals)
Covalent radius: 198±8 pm
Filling Orbital: 4f6
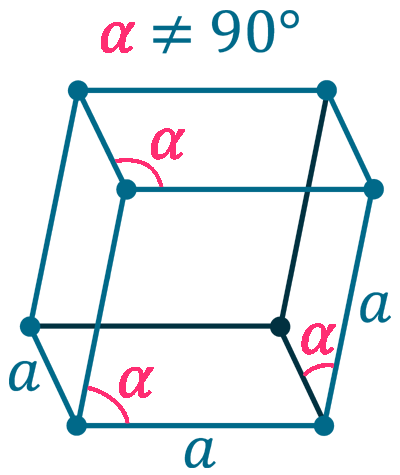
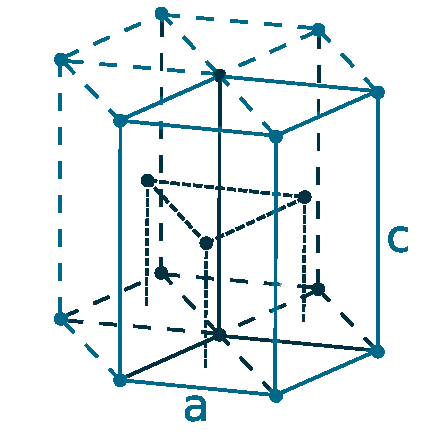
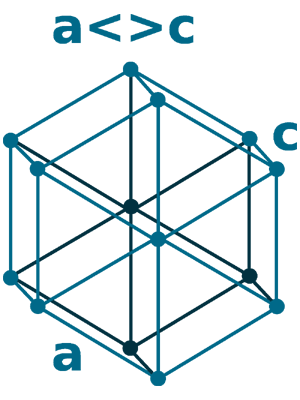
Crystal structure: Rhombohedral (At ambient temperature), Change to hexagonally close-packed (Above 731 oC), Transform metal into Body-centered cubic (Further Heating to 922 oC), Double hexagonal close-packed (Heating to 300 oC with 40 Kbar compression)
Grid parameters: a(H)=3.621 Å c(H)=26.25 Å
Attitude c/a: 7.25
Space Group Name: R_3m
Space Group Number: 166

Reactivity of Samarium
Electronegativity: pauling scale: 1.17
Valence: +3
Electron affinity: 50 kJ/mol
Nuclear Properties of Samarium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 7F0
Neutron cross section (Brans): 5900
Neutron Mass Absorption: 4.7
Isotopes: 144Sm 145Sm 146Sm 147Sm 148Sm 149Sm 150Sm 151Sm 152Sm 153Sm 154Sm
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 144Sm | 3.08 | 143.912 | Stable |
| 145Sm | Syn | – | 340 d |
| 146Sm | Syn | – | 6.8×107 y |
| 147Sm | 15.00 | 146.915 | 1.06×1011 y |
| 148Sm | 11.25 | 147.915 | 7×1015 y |
| 149Sm | 13.82 | 148.917 | Stable |
| 150Sm | 7.37 | 149.917 | Stable |
| 151Sm | Syn | – | 90 y |
| 152Sm | 26.74 | 151.920 | Stable |
| 153Sm | Syn | – | 46.284 h |
| 154Sm | 22.74 | 153.922 | Stable |
Chemical Reactions
Samarium burns readily at 150 oC to form samarium (lll) oxide:
4Sm + 3O2 → 2Sm2O3
Reacts slowly in cold water and rapidly in hot water (Produce Samarium hydroxide):
2 Sm (s) + 6 H2O (l) → 2 Sm(OH)3 (aq) + 3 H2 (g)
Dissolves readily in dilute sulfuric acid:
2 Sm (s) + 3 H2SO4 (aq) → 2 Sm3+ (aq) + 3 SO42−(aq) + 3 H2 (g)
The metal reacts with all Halogens to form Trihalides:
2 Sm (s) + 3 F2 (g) → 2 SmF3 (s) [white]
2 Sm (s) + 3 Cl2 (g) → 2 SmCl3 (s) [yellow]
2 Sm (s) + 3 Br2 (g) → 2 SmBr3 (s) [yellow]
2 Sm (s) + 3 I2 (g) → 2 SmI3 (s) [orange]
Samarium’s Alkyls and aryls are obtained through a metathesis reaction in tetrahydrofuran or ether: (R is hydrocarbon group and Me is Methyl)
SmCl3 + 3 LiR → SmR3 + 3 LiCl
Sm(OR)3 + 3 LiCH(SiMe3)2 → Sm{CH(SiMe3)2}3 + 3 LiOR
Samarium History
Naming: After the mineral Samarskite (Itself named after Vassili Samarsky-Bykhovets ‘Russian mining Engineer’)
Discovery: Lecoq de Boisbaudran (French chemist) (1878)
Samarium Uses
Samarium-cobalt (SmCo5) is used to make new permanent magnet material, which is much more powerful than iron magnets. It has an intrinsic coercive force as high as 2200 kA/m(KiloAmpere/meter).
Due to it’s Magnetism remain same at high temperature, It is also used in microwave applications. It enabled the miniaturization of electronic devices like headphones, and personal stereos etc.. Although, neodymium magnets are now more commonly used instead.
Along with other rare earths, Samarium is used for carbon-arc lighting for studio lighting and projection in motion picture industry.
Samarium is used to dope calcium chloride crystals for use in optical lasers.
Samarium oxide (Sm2O3) has been used in optical glass to absorb the infrared and as a neutron absorber in nuclear reactors and as a catalytic for the dehydration and dehydrogenation of ethyl alcohol (ethanol).
Biological role: It has Low-toxicity, But it should be handled with care.
Abundance of Samarium
Samarium is Found in the minerals monazite and bastnaesite, chiefly in Monazite (~2.8 %)
It commercially extracted by ion exchange and solvent extraction techniques.
Newly, electrochemical deposition has been used to extracted samarium from other lanthanides, by using a electrolyte solution of lithium citrate and a mercury electrode. It is Fast, simple, and highly specific way to extract or separate the rare-earths.
Samarium metal can also be produced by reducing the oxide with Lanthanum.
Annual world wide production is around 700 tons.
5×10-7% (In Universe)
1.7×10-5% (In Meteorites)
1×10-7% (In Sun)
0.0008% (In Earth’s Crust)
4.5×10-11% (In Oceans)
World’s Top 3 producers of Samarium
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders of Samarium
1) China
2) CIS Countries (inc. Russia)
3) USA
#samabium