77 Ir (Iridium)
Iridium is a hard, brittle, lustrous, dense, silvery-white with a slight yellowish cast metal of the platinum family, It is also a most corrosion resistant metal.
Iridium is not affected by air, water and acids.
It is niether attacked by any of the acids nor by aqua regia (mixture of nitric acid and hydrochloric acid), but is attacked by molten salts (NaCl & NaCN).

Identity
CAS Number: CAS74439-88-5
CID Number: CID23924
DOT Hazard Class: 4.1
DOT Number: 3089
CONTENT INDEX
Basic Properties of Zirconium
Pronunciation: i-rid-ee-am
Appearance: Silvery white
Mass Number: 192
Standard Atomic weight: 192.217 g/mol
Atomic number (Z): 77
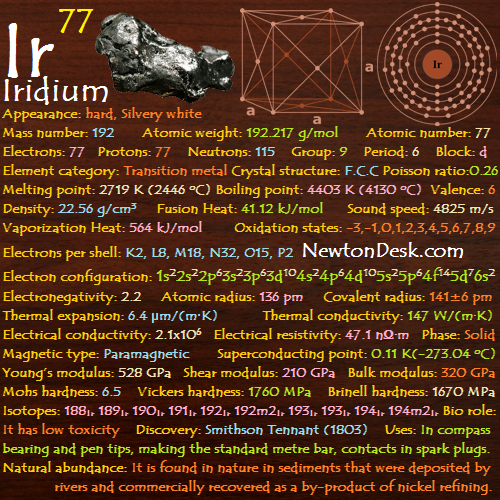
Electrons: 77
Protons: 77
Neutrons: 115
Period: 6
Group: 9
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M18, N32, O15, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f145d76s2
Thermal Properties of Iridium
Phase: Solid
Melting point: 2719 K (2446 oC, 4435 oF)
Boiling point: 4403 K (4130 oC, 7466 oF)
Debye temperature: 430 K (156.85 oC, 314.33 oF)
Fusion heat: 41.12 kJ/mol
Vaporization heat: 564 kJ/mol
Specific heat: 131 J/(kg K)
Molar heat capacity: 25.10 J/(mol.K)
Thermal expansion: 6.4 μm/(m∙K)
Thermal conductivity: 147 W/(m∙K)
Electrical properties of Iridium
Electrical conductivity: 21×106 S/m
A Electrical resistivity: 47.1 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 0.11 K (-273.04 oC, -459.47 oF)
Magnetic Properties of Iridium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +25.6×10-6 cm3/mol
Volume magnetic susceptibility: 0.0000377
Mass magnetic susceptibility: 1.67×10-9 m3/kg
Molar magnetic susceptibility: 0.321×10-9 m3/mol
Physical Properties of Iridium
Density: 22.56 g/cm3 (In solid) 19 g/cm3 (In Liquid at M.P)
Molar volume: 0.0000085203 m3/mol
Young’s modulus: 528 GPa
Shear modulus: 210 GPa
Mohs Hardness: 6.5
Bulk modulus: 320 GPa
Poisson ratio: 0.26
Vicker hardness: 1760-2200 MPa
Brinell hardness: 1670 MPa
Sound Speed: 4825 m/s
Atomic Properties of Iridium
Oxidation states: 9, 8, 7, 6, 5, 4, 3, 2, 1, 0, -1, -3
Valence Electrons: 5d7 6s2
Ion charge: Ir4+
Ionization energies: 1st: 544 kJ.mol 2nd: 1070 kJ/mol 3rd: 2260 kJ/mol
Ionic radius: 62.5 pm
Atomic radius: 136 pm (empirical)
Van der Waals: 202 Pm
Covalent radius: 141±6 pm
Filling Orbital: 5d7
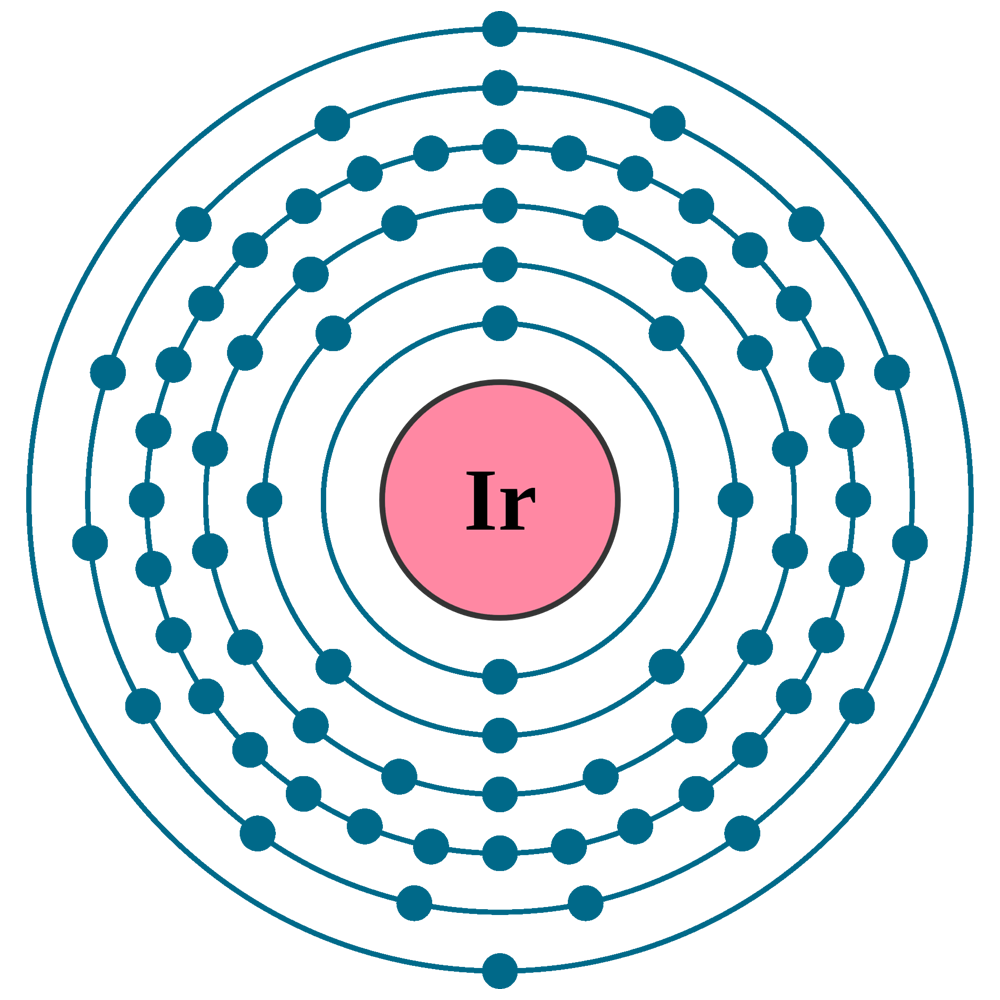
Crystal structure: Face centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 383.9, 383.9, 383.9 pm
Grid parameters: a=3.840 Å
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Iridium
Electronegativity: 1.17 (pauling scale)
Valence: +6
Electron affinity: 2.2 kJ/mol
Nuclear Properties of Iridium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 4F9/2
Neutron cross section (Brans): 425
Neutron Mass Absorption: 0.081
Isotopes: 188Ir 189Ir 190Ir 191Ir 192Ir 192m2Ir 193Ir 193mIr 194Ir 194m2Ir
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 188Ir | Syn | – | 1.73 |
| 189Ir | Syn | – | 13.2 d |
| 190Ir | Syn | – | 11.8 d |
| 191Ir | 37.3 | 190.962 | Stable |
| 192Ir | Syn | – | 73.827 d |
| 192m2Ir | Syn | – | 241 y |
| 193Ir | 62.7 | 192.961 | Stable |
| 193mIr | Syn | – | 10.5 d |
| 194Ir | Syn | – | 19.3 h |
| 194m2Ir | Syn | – | 171 d |
Chemical Reactions of Iridium
The metal reacts with oxygen, when heated:
Ir (s) + O2 (g) → IrO2 (s) [black] (Iridium (VI) oxide)
It doesn’t react with water under normal condition.
Reacts with fluorine, and forms highly corrosive Iridium (VI) fluoride, and also forms Iridium (V) fluoride (IrF5)4 when heated, which has the yellow tetrameric structure.
Ir (s) + 3 F2 (g) → IrF6 (s) [yellow] (Ir (VI) fluoride)
IrF6 (s) → (IrF5)4 [yellow]
Directly reacts with halogens under anhydrous (dry) conditions, and forms Iridium (III) halides:
2 Ir (s) + 3 F2 (g) → 2 IrF3 (s) [black] (Iridium (III) Fluoride)
2 Ir (s) + 3 Cl2 (g) → 2 IrCl3 (s) [red] (Iridium (III) Chlorine)
2 Ir (s) + 3 Br2 (g) → 2 IrBr3 (s) [red-brown] (Iridium (III) Bromide)
2 Ir (s) + 3 I2 (g) → 2 IrI3 (s) [dark brown] (Iridium (III) Iodide)
It is not reacts with acids, including aqua regia:
Iridium History
Naming: Latin: iris, iridis (rainbow).
Discovery & first isolation: Smithson Tennant (1803) in London, England
Iridium Uses
Iridium is the most corrosion-resistant material, where it is used in special alloys & forms an alloy with osmium, which is used for fountain pen tips & compass bearing.
Iridium alloys are used in making the standard meter bar (contains 90% platinum & 10% iridium), to make crucibles, and also used for the contacts in spark plugs because of its low reactivity & high melting point.
Nowadays demand for iridium comes mainly from the automotive industry, electronic industry, and chemical industry, where it is used to coat the electrodes in the chlor-alkali process (It is used to produce chlorine and sodium hydroxide (lye/caustic soda)), and in catalyst.
It is also used in pivot bearings, in scientific & other special equipment.
Biological role of Iridium
It has Low-toxicity, But Iridium-192 is dangerous like other radioactive isotopes, even it can increase the risk of cancer, because of high energy gamma radiation.
Abundance of Iridium
Iridium is one of the rarest elements on Earth, It occurs uncombined in nature with platinum and other metals of this family in alluvial deposits (Material deposited by rivers).
Commercially, It is recovered as a by-product from the nickel mining industry.
Asteroids & Meteors contain higher levels of iridium than the Earth’s crust, where due to the collision of large asteroid or meteor, a very thin layer of iridium has existed in the Earth’s crust,
Because of the collision of asteroid or meteors, a huge dust cloud deposited the iridium of all over the world, even some scientists think that this could be the same asteroid or meteor that wiped out the dinosaurs.
Annual world wide production is around 8 tons.
2×10-7% (In Universe)
5.4×10-5% (In Meteorites)
2×10-7% (In Sun)
4×10-8% (In Earth’s Crust)
World’s Top 3 producers of Iridium
1) South Africa
2) Russia
3) Zimbabwe
World’s Top 3 Reserve holders of Iridium
1) South Africa
2) Russia
3) USA
Iridium Price: Pure (99.995%) metal price is around $48,802 per KG (KiloGram).
#Iridium


