16 S (Sulfur Element)

Sulfur is abundant, pale yellow, odorless, tasteless, crystalline solid non metal, which is insoluble in water but soluble in carbon disulfide (CS2).
It is a good insulator.
In every state (liquid, solid, or gas), sulfur occurs in more than one allotropic form or modification.
The crystallography of sulphur is complex, which is depending on the specific conditions, where sulphur allotropes form several distinct crystal structures.
Highly Pure sulfur is commercially available with 99.999+% purities .
Organic compounds containing sulfur are very important, Like ammonium sulfate ((NH4)2SO4), Calcium sulfide (CaS), sulfur dioxide (SO2), carbon disulfide (CS2), hydrogen sulfide (H2S) etc.

Identity
CAS Number: CAS7440-34-9
CID Number: CID5362487
RTECS Number: RTECSWS4250000
CONTENT INDEX
Basic Properties of Sulfur
Alternative name: Sulphur (In British)
Allotropes: Rhombic Sulphur, Monoclinic Sulphur, Amorphous Sulphur
Pronunciation: Sal-far
Appearance: Lemon yellow sintered microcrystals
Mass Number: 32
Standard Atomic weight: 32.059, 32.075 g/mol
Atomic number (Z): 16
Electrons: 16
Protons: 16
Neutrons: 16
Period: 3
Group: 16
Block: p
Element category: Reactive nonmetal
Electrons per shell: K2, L8, M6
Electron configuration: 1s22s22p63s23p4

Thermal Properties of Sulfur
Phase: Solid
Melting point: 388.35 K (115.20 oC, 239.36 oF)
Boiling point: 717.7 K (444.55 oC, 832.19 oF)
Fusion heat: mono: 1.727 kJ/mol
Vaporization heat: mono: 45 kJ/mol
Specific heat: 705 J/(kg K)
Critical point temperature: 1314 K (1040.85 oC, 1905.53 oF)
Critical point pressure: 20.7 MPa (204.29 atm)
Molar heat capacity: 22.75 J/(mol.K)
Thermal conductivity: 0.205 W/(m∙K) (amorphous)
Electrical properties of Sulfur
Electrical conductivity: 1×10-15 S/m
A Electrical resistivity: 2×1015 Ω∙m (amorphous)
A Electrical type: Insulator
Magnetic Properties of Sulfur
A Magnetic type: Damagnetic
Magnetic susceptibility (xmol): (α) -15.5×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000122
Mass magnetic susceptibility: -6.2×10-9 m3/kg
Molar magnetic susceptibility: -0.199×10-9 m3/mol
Physical Properties of Sulfur
Density: α-2.07 g/cm3 , β-1.96 g/cm3 , γ-1.92 g/cm3 (In solid) 1.819 g/cm3 (In Liquid at M.P)
Molar volume: 0.000016357 m3/mol
Mohs Hardness: 2.0
Bulk modulus: 7.7 GPa
Atomic Properties of Sulfur
Oxidation states: -2, -1, 1, 2, 3, 4, 5, 6
Valence Electrons: 3s2 3p4
Ion charge: S2-
Ionization potential of an atom: 10.31
Ionization energies: 1st: 1000 kJ.mol 2nd: 2252 kJ/mol 3rd: 3357 kJ/mol
Ionic radius: 37 pm
Atomic radius: 127 pm
Van der Waals: 180 Pm
Covalent radius: 105±3 pm
Filling Orbital: 3p4
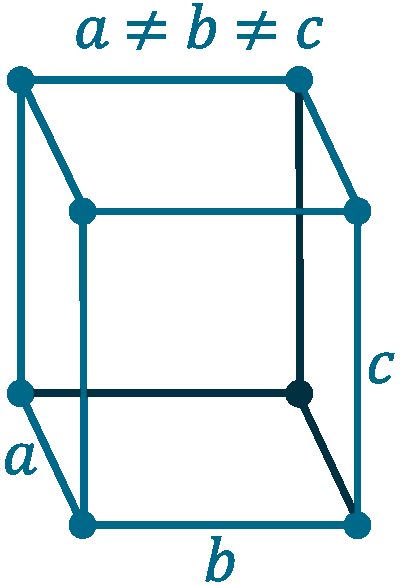
Crystal structure: Orthorhombic
Lattice angles: π/2, π/2, π/2
Lattice constant: 1043.7, 1284.5, 2436.9 pm
Grid parameters: a=10.437 Å b=12.845 c=24.369 Å
Space Group Name: Fddd
Space Group Number: 70
Reactivity of Sulfur
Electronegativity: 2.58 (pauling scale)
Valence: +6
Electron affinity: 200 kJ/mol
Nuclear Properties of Sulfur
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3P2
Neutron cross section (Brans): 0.52
Neutron Mass Absorption: 0.00055
Isotopes: 32S 33S 34S 35S 36S
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 32S | 94.99 | 31.971 | Stable |
| 33S | 0.75 | 32.972 | Stable |
| 34S | 4.25 | 33.969 | Stable |
| 35S | Trace | – | 87.32 d |
| 36s | 0.01 | 35.968 | Stable |
Chemical Reactions of Sulfur
It reacts with air, and forming sulfur dioxide & trioxide:
S (s) +O2 (g) → SO2 (g)
2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g)
It doesn’t reacts with water and dilute non-oxidizing acids, under normal conditions.
Reacts with excess fluorine, and forms sulfur (VI) fluoride:
S (s) + 3 F2 (g) → SF6 (s)
Reacts with excess chlorine, and forms sulfur (I) chloride & sulfur (II) chloride:
2 S (s) + Cl2 (l) → S2Cl2 (s)
S (s) + Cl2 (g) → SCl2 (s)
Sulfur reacts with excess bromine, and forming sulfur (I) bomide:
2 S (s) + Br2 (I) → S2Br2 (s)
It Doesn’t react with iodine.
Sulfur reacts with hot aqueous potassium hydroxide (KOH), and forms potassium sulfide & thiosulfate:
S8 (s) + 12 KOH (aq) → 4 K2S (aq) + 2 K2S2O3 (aq) + 6 H2O (l)
Sulfuric Acid:
2 S + 3 O2 + 2 H2O (l) → 2 H2SO4
Production:
Sulfur is produced from fossil resources, which obtained as hydrogen sulfide (H2S)
R-S-R + 2 H2 → 2 RH + H2S
After converted into elemental sulfur by the Claus process.
3 O2 + 2 H2S → 2 SO2 + 2 H2O
SO2 + 2 H2S → 3 S + 2 H2O
Sulfur History
Naming: Sanskrit sulvere, Latin: sulphurium (brimstone)
Discovery: Chinese (before 2000 BCE)
Recognized as an element: Antoine Lavoiser (1777)
Sulfur Uses
Sulfur is used in the vulcanisation of natural rubber, in fungicide, batteries, matches, fireworks, and in black gunpowder.
Most of the sulfur is used in the production of sulfuric acid (H2SO4), which is one of the most important elements used as an industrial raw material.
It is also extensively uses in the manufacture of phosphoric acid (H3PO4), to make phosphates (PO43-) for phosphatic fertilisers.
Mercaptans (methanethiol, CH4S) are a family of organosulfur compounds, where some are added to natural gas supplies because of their distinctive smell like garlic or rotten eggs, so that gas leaks can be detected easily.
Others are used in silver polish, and in the production of pesticides & herbicides (toxic substance to plants, which is used to destroy unwanted vegetation).
It is used to make sulfite (S2-O3), which are used to bleach paper and as preservatives for many foodstuffs like dried fruits.
Many detergents & surfactants are sulfate (S-2O4) derivatives.
Calcium sulfate (gypsum, CaSO4) is used in cement & plaster.
Sulfur is also used in making corrosion-resistant concrete, which has great strength & frost resistant.
Biological role of Sulfur
Sulfur is an essential element for all living things, which taken up as sulfate from the soil or seawater by plants & algae.
It is used to make two essential amino acids (Methionine & cysteine), which needed to make proteins, and also needed in some co-enzymes.
The average human (79 kg) contains 140 grams of sulfur and daily intake of about 1 gram a day, mainly in proteins.
Sulfur and sulfate (S-2O4) are Non-Toxic, However, hydrogen sulfide (H2S), carbon disulfide (CS2), and sulfur dioxide (SO2) are all toxic, and it should be handled with care.
Hydrogen sulfide (colorless, flammable with a “rotten egg” smell) is extremely hazardous (dangerous) gas, where H2S in small concentration can be metabolized, but in higher concentrations it can cause death quickly by respiratory paralysis (deadens the sense of smell).
Sulfur dioxide (SO2) is produced when unpurified oil & coal are burned, and due to this process sulfur dioxide can enter the atmosphere, where it reacts with oxygen to produce H2SO4 and H2SO3, which causes acid rain.
This acid rain can cause lakes to die, by releases toxic aluminium salts soluble, that effect the life of fish and other organisms.
Abundance of Sulfur
ASulfur often occurs naturally in volcanic areas, It is also widely found in many minerals including Galena (PbS), Iron Pyrites (FeS), Gypsum (CaSO4·2H2O), & Epsom salts (magnesium sulphate, MgSO4).
Commercially, Sulfur is recovered from wells (which sunk into the salt domes) by using the Frasch process, where heated water (super-heated steam) is forced into the wells to melt the sulfur, and when sulphur is melted underground, then pumped it up to the surface of the earth.
ASulfur also occurs in natural gas, petroleum crudes, and other Fossil resources, where sulfur is removed from these products by using various purification processes.
Amorphous (without a clearly defined form or shape) or “plastic” sulfur is obtained by fast cooling of the crystalline form.
According to X-ray studies, amorphous sulfur may have a helical structure with 8 atoms per spiral.
Crystalline sulfur seems to be made of rings, where each containing 8 sulfur atoms, which fit together to give a normal X-ray pattern.
A finely divided form of sulfur is also known as flowers of sulfur, which is obtained by sublimation.
Annual world wide production is around 75 million tonnes.
0.05% (In Universe)
4% (In Meteorites)
0.04% (In Sun)
0.042% (In Earth’s Crust)
0.093% (In Oceans)
0.2% (In Humans)




World’s Top 3 producers of Sulfur
1) China
2) USA
3) Canada
Sulfur Price
Pure (99.99%) element price is around $0.10-$0.20 per KG (KiloGram)
#Sulfur #Sulphur