19 K (Potassium Element)

Potassium is a silvery-white, soft metal, easily cut with a knife.
It is one of the most reactive & electropositive metals, and it is also the lightest element except lithium.
Because of its lightness, it floats into the water, where it reacts instantly to release hydrogen, which catches fire spontaneously & burns with a Lilac flame.
It rapidly oxidizes and tarnishes in air within minutes, so it must be stored in a mineral oil such as kerosene.
Potassium & its salts give a violet color to flames.

Identity
CAS Number: CAS7440-09-7
CID Number: CID5462222
DOT Hazard Class: 4.3
DOT Number: 2257
RTECS Number: RTECSTS6460000
CONTENT INDEX
Basic Properties of Potassium
Pronunciation: Pa-tas-ee-am
Appearance: Silvery gray
Mass Number: 39
Standard Atomic weight: 39.098 g/mol
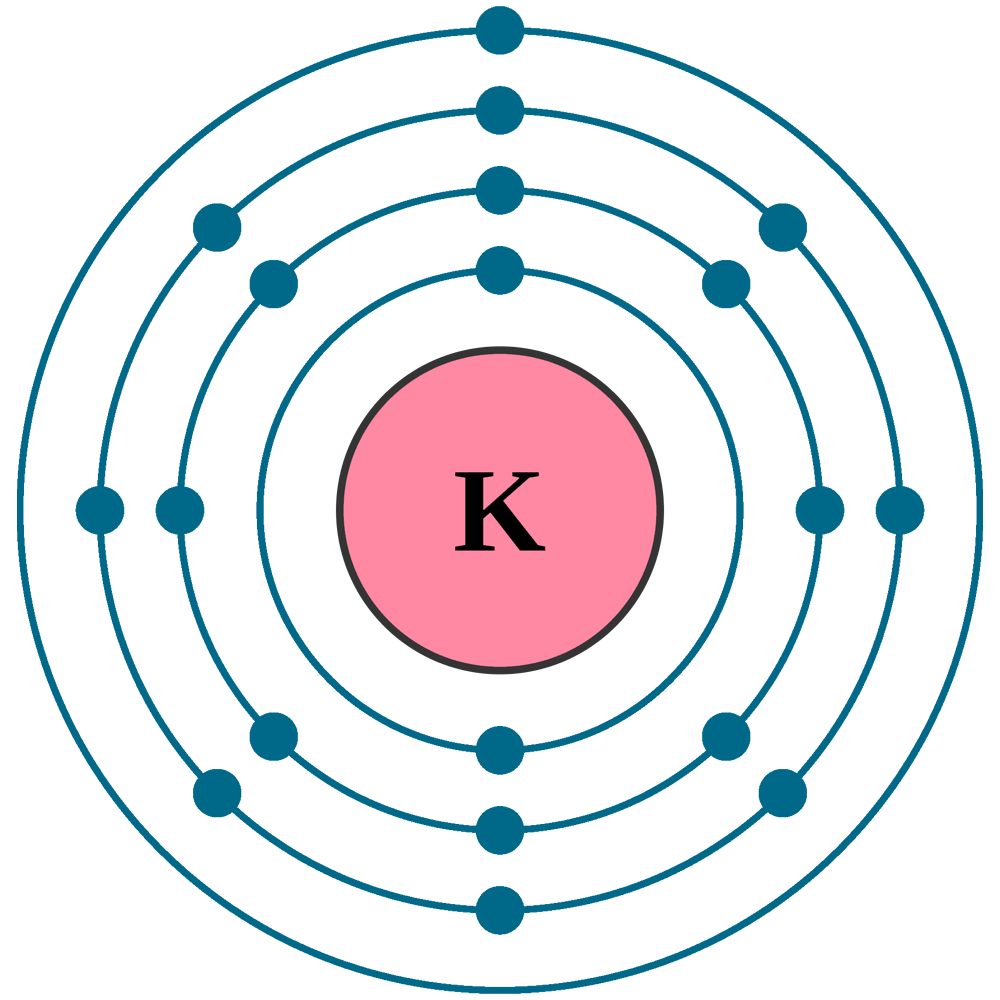
Atomic number (Z): 19
Electrons: 19
Protons: 19
Neutrons: 20
Period: 4
Group: 1
Block: s
Element category: Alkali metal
Electrons per shell: K2, L8, M8, N1
Electron configuration: 1s22s22p63s23p64s1
Thermal Properties of Potassium
Phase: Solid
Melting point: 336.7 K (63.5 oC, 146.3 oF)
Boiling point: 1032 K (759 oC, 1398 oF)
Debye temperature: 100 K (-173.15 oC, -279.67 oF)
Fusion heat: 2.33 kJ/mol
Vaporization heat: 80 kJ/mol
Specific heat: 757 J/(kg K)
Critical temperature: 2223 K (1949.85 OC, 3541.73 OF)
Critical Pressure: 16 MPa (157.9 Atm)
Molar heat capacity: 29.6 J/(mol.K)
Thermal expansion: 83.3 μm/(m∙K)
Thermal conductivity: 102.5 W/(m∙K)
Electrical properties of Potassium
Electrical conductivity: 14×106 S/m
A Electrical resistivity: 72 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Potassium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +20.8×10-6 cm3/mol
Volume magnetic susceptibility: 0.00000574
Mass magnetic susceptibility: 6.7×10-9 m3/kg
Molar magnetic susceptibility: 0.262×10-9 m3/mol
Physical Properties of Potassium
Density: 0.862 g/cm3 (In solid) 0.828 g/cm3 (In Liquid at M.P)
Molar volume: 0.00004568 m3/mol
Young’s modulus: 3.53 GPa
Shear modulus: 1.3 GPa
Mohs Hardness: 0.4
Bulk modulus: 3.1 GPa
Brinell hardness: 0.363 MPa
Sound Speed: 2000 m/s
Atomic Properties of Potassium
Oxidation states: -1, +1
Valence Electrons: 4s2
Ion charge: K+
Ionization potential of an atom: 4.32
Ionization energies: 1st: 418 kJ.mol 2nd: 3052 kJ/mol 3rd: 4420 kJ/mol
Ionic radius: 138 pm
Atomic radius: 227 pm (empirical)
Van der Waals: 275 Pm
Covalent radius: 203±12 pm
Filling Orbital: 4s1
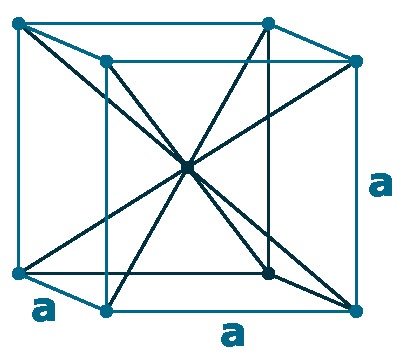
Crystal structure: Body centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 533.2, 533.2, 533.2 pm
Grid parameters: a=5.332 Å
Space Group Name: lm_3m
Space Group Number: 229
Reactivity of Potassium
Electronegativity: 0.82 (pauling scale)
Valence: +1
Electron affinity: 48.4 kJ/mol
Nuclear Properties of Potassium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2S1/2
Neutron cross section (Brans): 2.1
Neutron Mass Absorption: 0.0018
Isotopes: 39K 40K 41K
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 39K | 93.258 | 38.962 | Stable |
| 40K | 0.012 | 39.964 | 1.248×109 y |
| 41K | 6.730 | 40.965 | Stable |
Chemical Reactions of Potassium
It reacts slowly with air under normal condition, But when heated, it oxidizing to form potassium superoxide:
K (s) + O2 (g) → KO2 (s) [orange]
Reacts violently with water, and forming a colourless solution of potassium hydroxide & hydrogen gas:
2 K (s) + 2 H2O (l) → 2 KOH (aq) + H2 (g) ↑
Reacts with Halogens, and forms potassium halides:
2 K (s) + F2 (g) → 2 KF (s) (potassium fluoride)
2 K (s) + Cl2 (g) → 2 KCl (s) [white] (potassium chloride)
2 K (s) + Br2 (g) → 2 KBr (s) (potassium bromide)
2 K (s) + I2 (g) → 2 KI (s) (potassium iodide)
The metal dissolves readily in dilute sulphuric acid, and forming potassium ions & hydrogen gas:
2 K (s) + H2SO4 (aq) → 2 K+ (aq) + SO42- (aq) + H2 (g)
It reacts with hydrogen, and forming potassium hydride:
2 K (s) + H2 (g) → 2 KH (s)
Potassium History
Naming: From potash (pot ash) & K symbol from latin: kalium (meaning alkali)
Discovery and first isolation: Humphry Davy (1807) in London, England
Potassium Uses
The greatest demand for potassium compounds (potash) has been in its use for fertilizers.
Many other potassium salts are of great importance, including the hydroxide (KOH), nitrate (KNO3), carbonate (K2CO3), iodide, chloride (KCl), bromide (KBr), cyanide (KCN), chlorate (KClO3) and sulfate (K2SO4).
Alloy of sodium & potassium (NaK) is used as a heat-transfer medium.
Potassium hydroxide (KOH) is a strong base, which is used in industry to neutralize weak & strong acids, to control pH (pouvoir hydrogen/ power of hydrogen) and to manufacture potassium salts.
It is also used to saponify (turn into detergent or liquid soap by reaction with an alkali) fats & oils, in industrial cleaners, and in hydrolysis reactions like esters.
Potassium nitrate (KNO3) is the oxidant (ability to oxidize other substances) in gunpowder (black powder) and an important agricultural fertilizer, It is obtained by Haber process.
Potassium carbonate (K 2CO3 or potash) is used in the manufacture of soap, glass, fluorescent lamps, color TV tubes, textile dyes & pigments.
About 90% Potassium chloride (KCl) is used in agriculture fertilizers, It is also used in US for Lethal injection (injecting drugs into a person for immediate death) executions.
Potassium bromide (KBr) was formerly used as a sedative (tranquilliser, promoting calm or inducing sleep) and in photography.
Potassium cyanide (KCN) is used in industries to dissolve copper & precious metals, in particular silver & gold by forming complexes.
Include gold mining, electroforming, & electroplating of these metals, it is also used in organic synthesis to make nitriles (Organic compound).
Potassium chlorate (KClO3) is used in matches and explosives.
Potassium sulfate (K2SO4) is commounly used in fertilizers, but it doesn’t contain chloride, which can be harmful to some crops, that’s why it prefered only for chloride-sensitive crops (include tobacco and some fruits & vegitables), which needing higher sulfur content.
Biological role of Potassium
Potassium ions is essential to life of all living spices, and its ions are found in all cells, where It is important for maintaining fluid & electrolyte balance.
Plant cells are particularly rich in potassium ions, which they get from the soil.
Harvests are taken every year from Agricultural land, which needs to have its potassium replenished (fill up again) by adding potassium-based fertilizers.
The average human (around 60 kg adult) consumes up to 7 grams of potassium in a day, and stores about 120 grams in the body cells.
A normal healthy diet contains enough potassium, but some foods such as sardines (small oily fish), raisins (dried grape), instant coffee, nuts, potatoes and chocolate have above average potassium content.
The naturally occurring isotope potassium-40 is radioactive, although it has mild radioactivity, and it may be one natural cause of genetic mutation (permanent alteration in the DNA sequence) in humans.
Abundance of Potassium
Potassium is the 7th most abundant metal in the Earth’s crust (about 2.4% by weight).
Most potassium minerals are found in igneous rocks and are Insoluble , that’s why the metal is very difficult to obtain from these minerals.
Certain minerals, such as Sylvite (potassium chloride, KCl), Carnallite (hydrated potassium magnesium chloride, KCl.MgCl2·6(H2O)), Langbeinite (potassium magnesium sulfate, K2Mg2(SO4)3), and Polyhalite (hydrated sulfate of potassium, calcium and magnesium, K2Ca2Mg(SO4)4·2H2O) are found in ancient lake & sea beds (bottom of the ocean), where these minerals are extensive deposits from which potassium and its salts can readily be obtained.
Large deposits of potash, found at a depth of approx 3000 feet in Saskatchewan, Canada.
Potassium is also found in the ocean but only in smaller amounts compared to sodium.
Annual world wide production of Potash (potassium carbonate) is around 9,000,000 tons.
0.0003% (In Universe)
0.07% (In Meteorites)
0.0004% (In Sun)
1.5% (In Earth’s Crust)
0.042% (In Oceans)
0.2% (In Humans)





World’s Top 3 producers of Potassium
1) Canada
2) Russia
3) Belarus
World’s Top 3 Reserve holders of Potassium
1) Canada
2) Russia
3) Belarus
Potassium Price
Pure (99.99%) metal price is around $1000 per KG (KiloGram)
#Potassium


