64 Gd (Gadolinium)

It is a soft, shiny, ductile, silvery white, has a metallic lusture,
The metal is relatively stable n dry air, but an oxide film forms in moist air.
Godolinium reacts slowly with water and is dissolves in dilute acid.

Identity
CAS Number: CAS7440-54-2
CID Number: CID23982
RTECS Number: RTECSLW3850000
CONTENT INDEX
Basic Properties of Gadolinium
Pronunciation: Gad-o-lee-nee-am
Appearance: Silvery white
Mass Number: 157
Standard Atomic weight: 157.25 g/mol
Atomic number (Z): 64
Electrons: 64
Protons: 64
Neutrons: 93
Period: 6
Block: f
Element category: Lanthanide
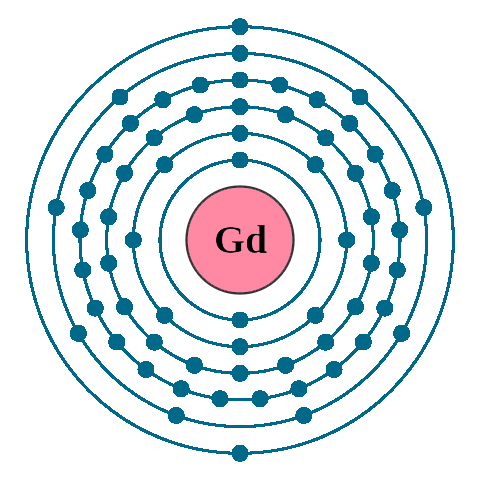
Electrons per shell: K2, L8, M18, N25, O9, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f75d16s2
Thermal Properties of Gadolinium
Phase: Solid
Melting point: 1585 K (1312 oC, 2394 oF)
Boiling point: 3273 K (3000 oC, 5432 oF)
Fusion heat: 10.05 kJ/mol
Vaporization heat: 301.3 kJ/mol
Molar heat capacity: 37.03 J/(mol.K)
Thermal expansion: α, poly: 9.4 μm/(m∙K)
Thermal conductivity: 10.6 W/(m∙K)
Electrical properties of Gadolinium
Electrical conductivity: 0.77×106 S/m
a Electrical resistivity: α, poly: 1.310 μΩ∙m
a Electrical type: Conductor
Critical point (Superconducting point): 1.083 K
Magnetic Properties of Gadolinium
Magnetic type: Paramagnetic
Curie point: 292 K (above which Ferromagnetism vanishes)
Magnetic susceptibility (xmol): +755,000×10-6 cm3/mol
Physical Properties of Gadolinium
Density: 7.90 g/cm3 (In solid) 7.4 g/cm3 (In Liquid)
Molar volume: 0.0000199 m3/mol
Young’s modulus: α form: 54.8 GPa
Shear modulus: α form: 21.8 GPa
Mohs Hardness: 5.3
Bulk modulus: α form: 37.9 GPa
Poisson ratio: α form: 0.259
Vicker hardness: 510-950 MPa
Sound Speed: 2680 m/s
Atomic Properties of Gadolinium
Oxidation states: 3,2,1
Valence Electrons: 4f7 5d1 6s2
Ion charge: Gd3+
Ionization energies: 1st: 593.4 kJ.mol 2nd: 1170 kJ/mol 3rd: 1990 kJ/mol
Ionic radius: 93.8 pm
Atomic radius: 237 pm (Van der Waals)
Covalent radius: 196±6 pm
Filling Orbital: 4f7
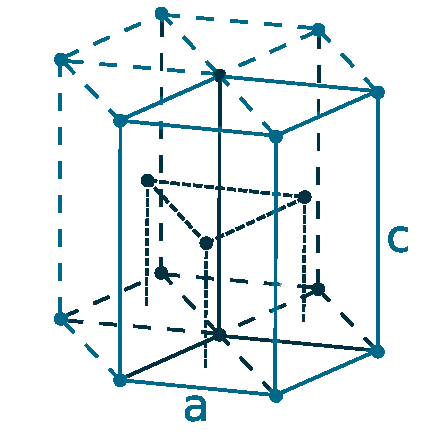
Crystal structure: Hexagonal close-packed (α form At room temperature), Body-centered cubic (above 1235 oC)
Lattice angles: π/2, π/2, π/3
Lattice constant: 363.6, 363.6, 578.3 pm
Grid parameters: a=3.636 Å, c=5.783 Å
Attitude c/a: 1.590
Space Group Name: P63/mmc
Space Group Number: 194

Reactivity of Gadolinium
Electronegativity: pauling scale: 1.2
Valence: +3
Electron affinity: 50 kJ/mol
Nuclear Properties of Gadolinium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 9D2
Neutron cross section (Brans): 49000
Neutron Mass Absorption: 7.3
Isotopes: 148Gd 150Gd 152Gd 154Gd 155Gd 156Gd 157Gd 158Gd 160Gd
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 148Gd | Syn | – | 75 y |
| 150Gd | Syn | – | 1.8×106 y |
| 152Gd | 0.20 | 151.920 | 1.08×1014 y |
| 154Gd | 2.18 | 153.921 | Stable |
| 155Gd | 14.80 | 154.923 | Stable |
| 156Gd | 20.47 | 155.922 | Stable |
| 157Gd | 15.65 | 156.924 | Stable |
| 158Gd | 24.84 | 157.924 | Stable |
| 160Gd | 21.86 | 159.927 | Stable |
Chemical Reactions
Gadolinium tarnishs quickly in moist air and form a Gadolinium (lll) oxide:
4 Gd + 3 O2 → 2 Gd2O3
Reacts slowly with cold water and quickly with hot water (form Gadolinium hydroxide and hydrogen gas):
2 Gd + 6 H2O → 2 Gd(OH)3 + 3 H2
The metal reacts with all Halogens to form Gadolinium (lll) halides:
2 Gd (s) + 3 F2 (g) → 2 GdF3 (s) [white] (Gadolinium (lll) fluoride)
2 Gd (s) + 3 Cl2 (g) → 2 GdCl3 (s) [white] (Gadolinium (lll) chloride)
2 Gd (s) + 3 Br2 (g) → 2 GdBr3 (s) [white] (Gadolinium (lll) bromide)
2 Gd (s) + 3 I2 (g) → 2 GdI3 (s) [yellow] (Gadolinium (lll) iodide)
Dissolves readily in dilute sulfuric acid to form Solutions containing Gadolinium (lll) ions (Colourless):
2 Gd + 3 H2SO4 + 18 H2O → 2 [Gd(H2O)9]3+ + 3 SO42− + 3 H2
Gadolinium History
Naming: After the mineral Gadolinite (Itself named after Johan Gadolin)
Discovery: Jean Charles Galissard de Marignac (1880)
Gadolinium Uses
It is excellent in absorbing neutrons, so It is used in the core of nuclear reactors.
Gadolinium is used to make garnets for used in microwave applications and Its compounds are used as phosphors in color television sets.
Gadolinium ethyl sulfate has extremely low noise characteristics and It may use in duplicating the performance of amplifiers, such as the maser (A device using the stimulated emission of radiation by excited atoms to amplify).
The metal has unusual superconductive properties.
In alloys, As little as 1% gadolinium is use to improve the workability of iron and chromium alloys, and their resistance to high temperatures and oxidation.
It’s alloys are used for making magnets, electronic components (recording heads for video recorders) and data storage disks.
Its compounds are useful in MRI (Magnetic Resonance Imaging), for diagnosing cancerous tumours.
Biological role: It is Low-toxic, But it should be handled with care.
Abundance of Gadolinium
Gadolinium is chiefly Found in the minerals monazite and bastnaesite.
It can be commercially extracted by ion exchange and solvent extraction.
Gadolinium metal is also produced by reducing the anhydrous fluoride with calcium metal.
Annual world wide production is around 400 tons.
2×10-7% (In Universe)
2.3×10-5% (In Meteorites)
2×10-7% (In Sun)
0.00052% (In Earth’s Crust)
7×10-11% (In Oceans)
World’s Top 3 producers of Gadolinium
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders of Gadolinium
1) China
2) CIS Countries (inc. Russia)
3) USA
#Gadolinium