Noble Gases On The Periodic Table
CONTENT INDEX
About Noble Gases
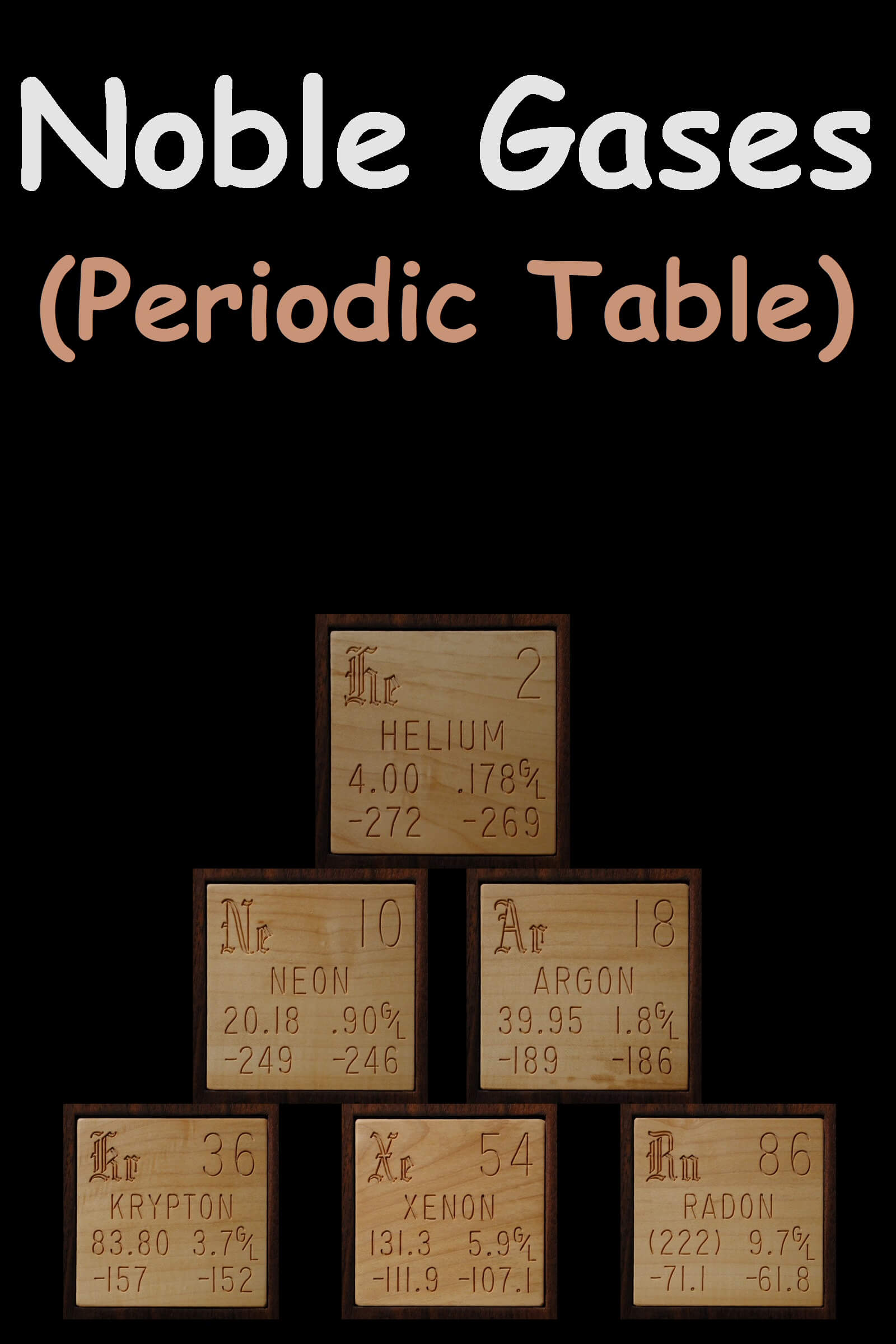
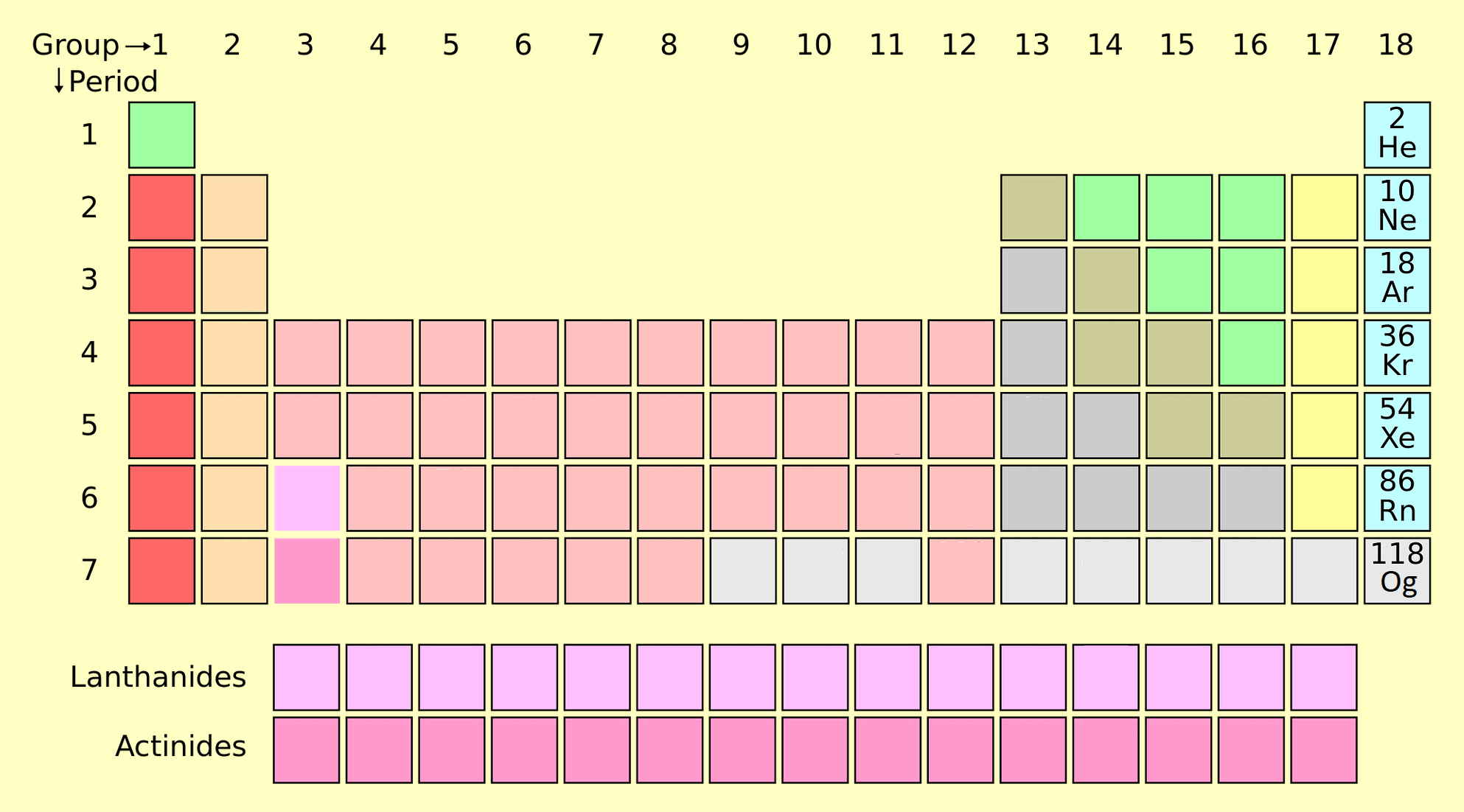
Noble gases are located as group 18 elements on the periodic table. These elements include Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn) and Oganesson (Og).
- These elements having a full valence shell of electrons.
- Helium has two valence shell electrons while each of the other noble gases has eight valence electrons.
- All Nobel gases have full valence shells which makes them very stable elements.
- They cannot not react with other elements therefore these are called the inert or unreactive gases. However, some noble gases can actually react to form certain compounds, that’s why this group is referred to as noble gases.
- All the noble gases are colorless, odorless, tasteless and monoatomic that means they exist as single atoms.
- When we moves down, the group number of electron shells increases, hence, the size of the atom will be increase.
- The atom size also affects the boiling point, This boiling point rises because the intermolecular forces between larger atoms with more electrons are greater than the force between smaller atoms with fewer electrons.
To know more about it, we have to study about this topic in details like where are they found and what’re their physical and chemical properties, Then I hope you will understand about it properly. So, let’s study about their occurrence, physical and chemical properties.
Occurrence:
- The noble gas elements are found in the periodic table between the halogen elements and the alkali metals or last column of right side.
- Noble gases are found in atmosphere to extent about 1% by volume.
- The abundance of noble gases decreases as their atomic numbers increases.
- Helium is the most abundant element in the earth after hydrogen. And helium is present in the air while other noble gases are found in earth’s atmosphere. So, the percentage of noble gases in air is as He (0.0005%), Ar (0.94%), Xe (0.00001%), Ne (0.015%), Kr (0.0001%).
- Radon is not found in atmosphere because it is radioactive element.
General Characteristics of Noble Gases
- They all are gases at room temperature.
- Their atomic size increases by increasing atomic number due to increase in number of shells when we go from top to bottom in a group.
- These gases are odorless, colorless, tasteless with extremely low melting points and boiling points. Further, B.P. and M.P. are increases by increasing atomic number.
- These have very high ionization enthalpy and negligible electron affinity. Hence do not show tendency to gain or lose electron.
- They obey octet rule (ns2, np2). It means they have 8 electrons in their outer most shell and their electronic configuration is (ns2, np2) except He (1s2) because their outer most shell contain only 2 electrons.
- These can be liquified due to London dispersion forces.
- These are almost chemically inert but form few compounds also. For example- compounds of helium are called helides. These are formed at low pressure in presence of Mercury (Hg), Tungsten (W) etc. By sparking He. For example:- HgHe2, HgHe10, FeHe etc. The compounds are formed due to adsorption of He on metal surface. So, these are not considered as true compounds.
- Noble gas is also form ‘’clatherates’’.
- Clatherates are cage compounds in which atoms or molecules a trapped in a cage of covalently bonded atoms but not directly bonded to them. Ar, kr and xe form solid compounds with certain molecules. Example- phenol (C6H5O), nete-hydroquinone (C6H4(OH)2), quinol (C6H6O2) under pressure.
- Xe by donating pair of electrons form unstable compounds. For example:- ArBf3, Ar2Bf3
- They are form compound with water at low temperature and high pressure known as hydrates. For example:- Ar.6H2O, Kr.6H2O, Xe.6H2O etc.
- Xe combine with most electronegative f and O to form XeF2, XeF4, XeOF4, XeO3
- Powerful oxidizing agents can react with noble gases.
For example- Xe + PtF6 → XePtF6
Properties of Noble Gas
Physical Properties of Noble Gases
- As we go down the group atomic size will be increases due to increasing in number of shells.
- They have low melting and boiling points.
- Ionization energy- noble gas have high ionization energy as compared to other elements.
- Electron affinity- Electron affinity of noble gas is zero.
- Liquefication- As we go down the group, liquefication will be increases.
- Adsorption – when we moving down the group adsorption capacity will increase.
Chemical Properties of Noble Gases
- As we moving down the group reactivity will be increases.
- Formation of compounds through coordination bond.
- All xenon fluorides are strong oxidizing agents.
- XeF2 +H2 →2HF+Xe
- XeF4+H2 →4HF+Xe
- XeF6 +H2→6HF+Xe
- Reaction with water:
2XeF2 + 2H2O → O2 + 2Xe + 4HF - Xenon form two types of oxides are XeO3 and XeO4. These are prepared by the following reactions:
Hydrolysis of XeF6 can give XeO3
XeF6 + 3H2O →XeO3+ 6HF
Uses of Noble Gases
There are some uses of noble gases in our daily life.
Uses of Helium
- Helium is most commonly used to fill up balloons. Because it is too lightweight, It allows balloons to float high.
- It is useful to control breathing, like emphysema and asthma.
- It helps MRI scan to produce accurate images of internal structure of the body. In most cases, it combines with oxygen to reach the lungs.
- It is used to make and obtain powerful superconducting magnets which are an important part of modern ‘’NMR’’ spectroscopy and MRI diagnosis.
- It is also used in gas cooled nuclear reactor.
Uses of Neon
- It is used in vacuum tubes.
- It is also used in fluorescent lights to display advertisements.
- Neon lights are used in botanical gardens and in green houses.
Uses of Argon
- Argon (Ar) is used mainly to provide an inert atom in high temperature extraction process.
- Used in Metal welding
- To fill the light bulb.
- It is also used in the laboratory.
Uses of Kr and Xe
- They are used in light bulbs designed to special purposes.
Uses of Radon
- Radon is used in radiotherapy to treat cancer.
- It also can be used to track movements of air masses by atmospheric scientists.
- Used to treat arthritis.
- In X-ray photography.