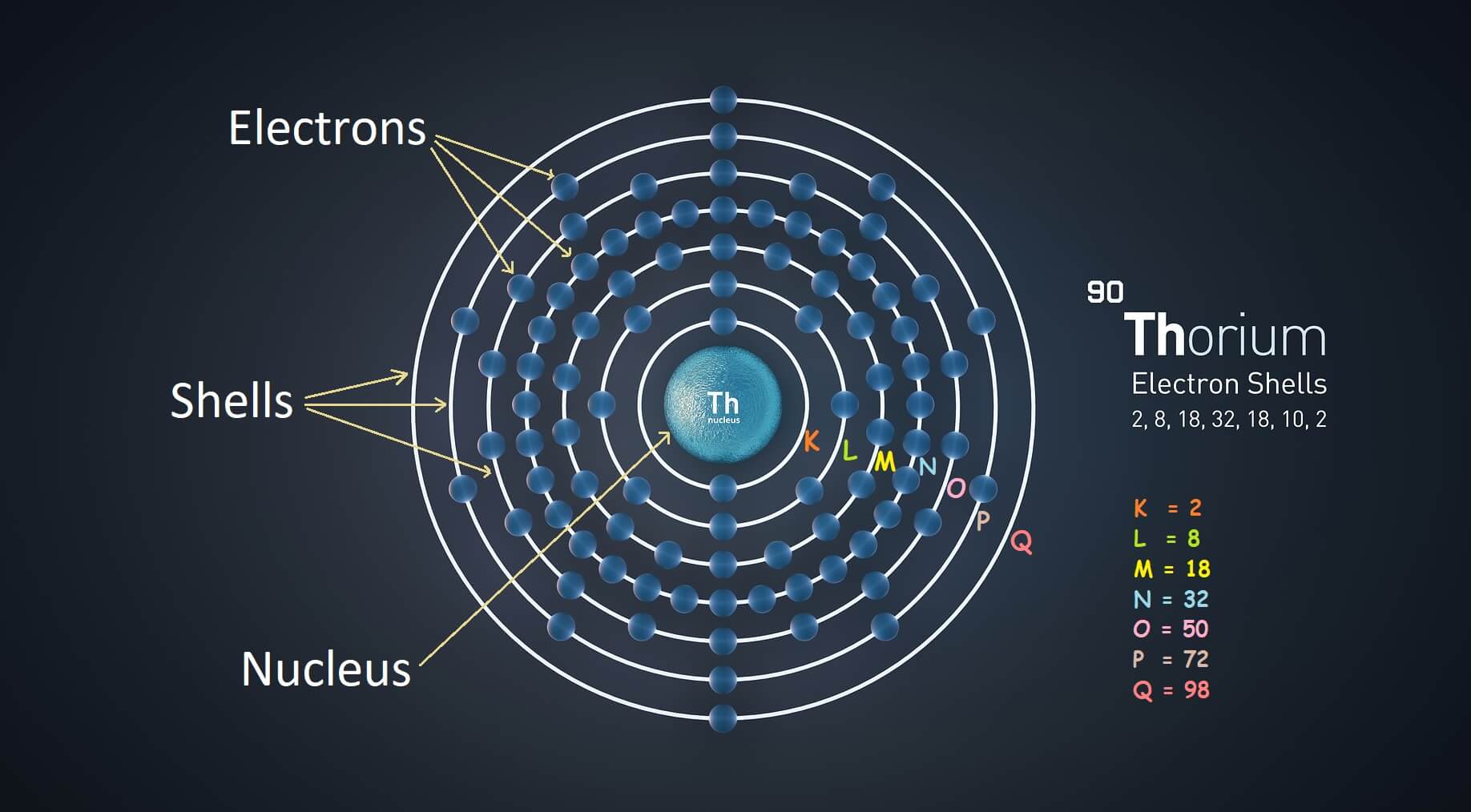
Electron Configuration of Elements In Periodic Table
CONTENT INDEX
Introduction
The nucleus contains neutrons and protons at the center of the atom. But what about electrons, how are they present within the atom. They don’t hang around like a bunch of grapes. We already know that they revolve around the nucleus but are they revolving randomly? These electrons can fit in single orbit? We don’t know about these facts. So, to understand about the arrangements of electrons here we have to study about the electron configuration. Now we will discuss about electron configuration and other facts which is related to electrons.
Electron Configuration
A layered arrangement of shells, where a specific number of electrons orbit around the nucleus. This arrangement is known as electronic configuration.
- Shells of an atom have a layered arrangement.
- Each shell has a specific number of electrons that orbit around the nucleus.
Bohr-bury Scheme
A scientists named Charles burry gave a set of simple rules that made it very easy to understand, how electrons are arranged in the Bohr’s atomic model. This scheme of electron arrangement is known as Bohr-bury scheme of electron arrangement.
Let’s study about them one by one. Look at the orbits or shells around the nucleus as proposed by Bohr. Now that we want to fill these orbits with electrons. where do you think we should start from the innermost shell or from the outermost shell? To understand about this, we need to understand a bit more about atomic orbits.
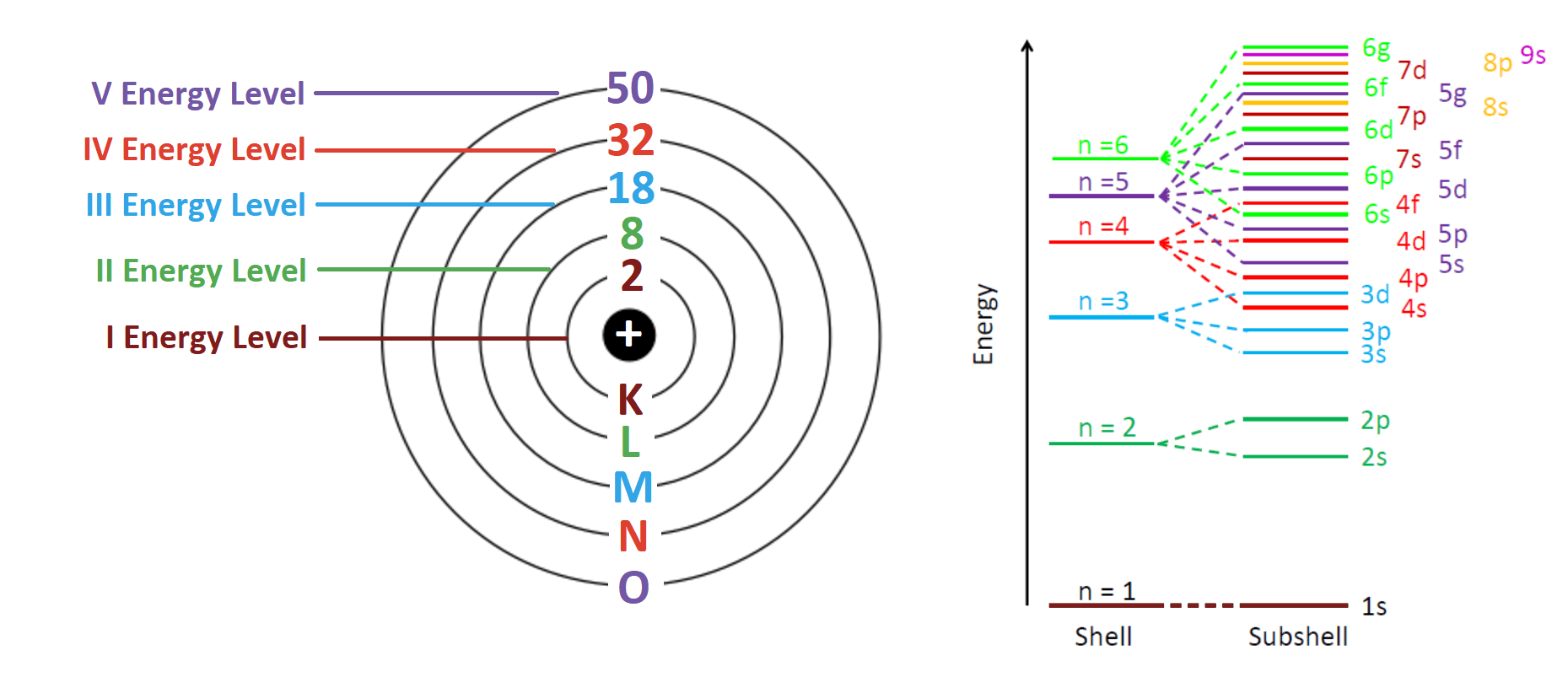
You know that the shells are also called energy levels as we have fixed energy. This energy is the least for the innermost orbit and as we move out energy keeps increasing, which in fact brings us to our first rule.
Rule 1: An electron will always fill an orbit or shell with lower energy and then occupy the higher energy ones.
This means that first we fill the K shell then L and then M and so on. Now we know the order electrons will be arranged. Now the question is how many electrons can be placed in each shell?
How many electrons can be placed in each shell?
Each orbit can take the same number of electrons? Or does the number of electrons vary with every orbit? Now the study about this. So, Bohr and bury gave the actual number of electrons as a shell can hold which in fact brings us to second rule.
Rule 2: Only a fixed number of electrons can be filled in each orbit, depending on the orbit number. This can be determined by a simple formula = 2n2. Where ‘n’ is the orbit number. Hence this rule is also known as 2n2 rule.
| Shell | Shell number(n) | Number of electrons(2n2) |
| K shell | 1 | 2×12= 2 |
| L shells | 2 | 2×22=8 |
Rule 3: The maximum electrons that the outermost shell of an atom can possess is 8. It also known as octet rule. It is derived from “octa’’, meaning eight. Eight is considered as a magic number for the atom. But why?
So, the answer is eight electrons in the outermost shell makes the atoms stable. This outermost shell is also known as the valence shell. The electrons that are present in valence shell is known as valence electrons.
For examples:
Hydrogen – It has one electron. So, it will obviously go in the first shell that is K shell.
Helium – It has two electrons. So, both of electrons will accommodate in K shell.
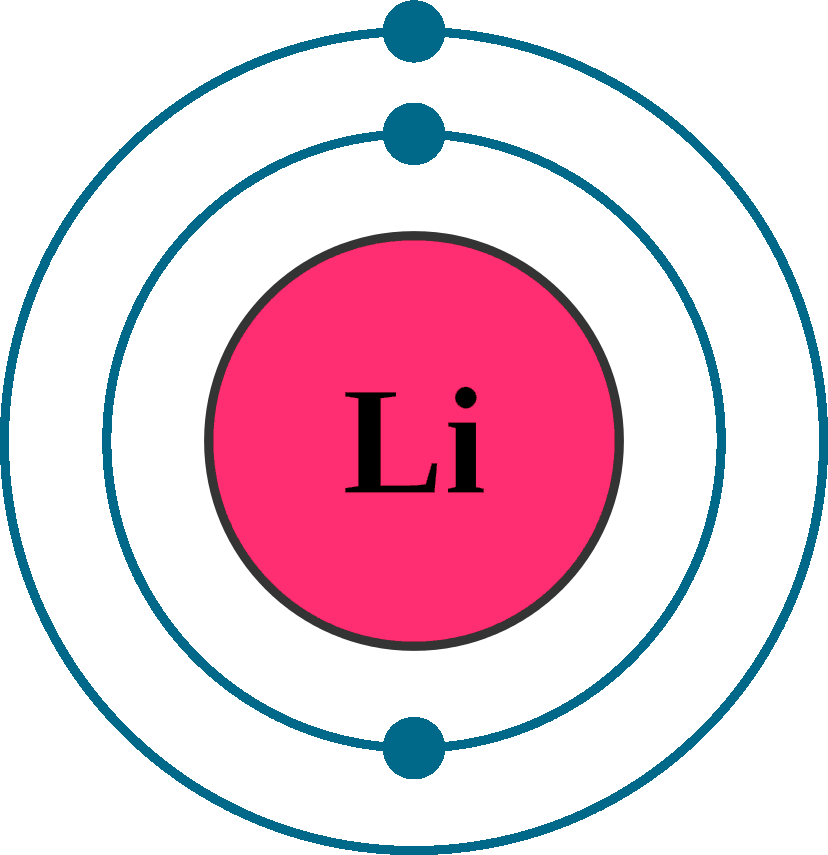
Lithium – It has three electrons. First two electrons will go in K shell, and third one will go in the second shell that is L shell.
Three Principles of Electron Configuration
There are three principles which are following:
1) Aufbau’s principle- According to this principle, electrons are filled in the increasing order of energy. However, the orbital having lower ‘n’ value will be occupied first, in case any two orbitals have the same (n+1) value.
Sequence of energy level- 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p and so on.
2) Hund’s rule – According to this rule, in the same energy orbitals, electrons filled first in the same spin then goes to pairing.
For example- Oxygen has 8 electrons. So,
- The first electron goes into the 1s orbital of the K shell.
- The second electron pairs with the first electron in the same 1s orbital.
- Similarly, the third and fourth electrons occupy the 2s orbital of the L shell.
- The fifth electron enters one of the three 2p orbitals of the L cell. Let that one 2px since the 3p orbitals 2px, 2py, 2pz are degenerate.
- The sixth electron enters into 2py or 2pz but not into 2px. Let’s say it goes into 2p1 since 2pz is a degenerate orbital.
- The 7th electron goes into 2pz instead of pairing up with the electron to 2px or 2py. Now since all three sub orbitals have one electron each.
- The eight-electron can pair with any of the 3 electrons in 2px, 2py and 2pz.
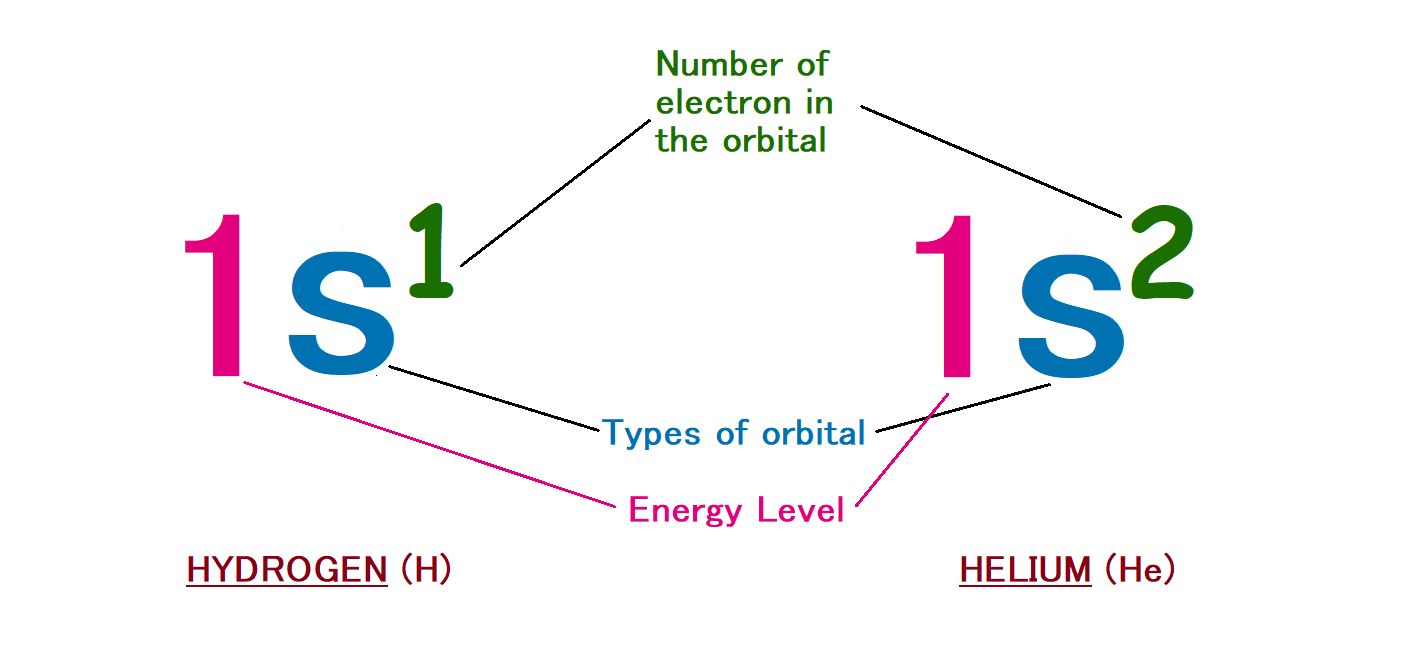
- So, the electronic configuration of oxygen can be written as 1s22s22p4.
3) Pauli exclusion principle – According to this principle, it is impossible that all four quantum numbers [n, l, m, s] are same for any two electrons are present in an orbital. Because of this rule a single orbital can have only 2 electrons.
Exception
There are two main exceptions to electronic configuration
Chromium and Copper – In these cases, a fully or half-full d sublevel is more stable than a partially filled d sublevel, so an electron from the 4s orbital is excited and enters into the 3d orbital. So, we can say that these two are exceptions, because it is easier for them to remove a 4s electron and bring it into the 3d subshell, which will give them a half-full or fully-full subshell, creating more stability.
Since the 4s orbital has a lot of electronic repulsion, it easily donates an electron to the nearby 3d orbital. A half or fully full orbital will be more stable due to symmetry.
Now I hope you understand about the electronic configuration and principles which is related to it.