Basic Concept of Atoms and Molecules
CONTENT INDEX
History of Atoms and Molecules:
As we know that everything around us is matter. Everything that has mass and occupy space or volume is matter. there are three states of matter:
1. Solid
2. Liquid
3. Gas.
There are some Ancient thoughts about Atoms and Molecules: philosopher mahrishi Kanad said that padarth (matter) is made up of small unit which is called as parmanu (atom). But after sometimes two Greek philosopher Democritus and Leucippus who gave name this parmanu as atoms. Which is Greek word.
What’s the Concept of an Atom?
- Atoms are the basic building blocks of matter.
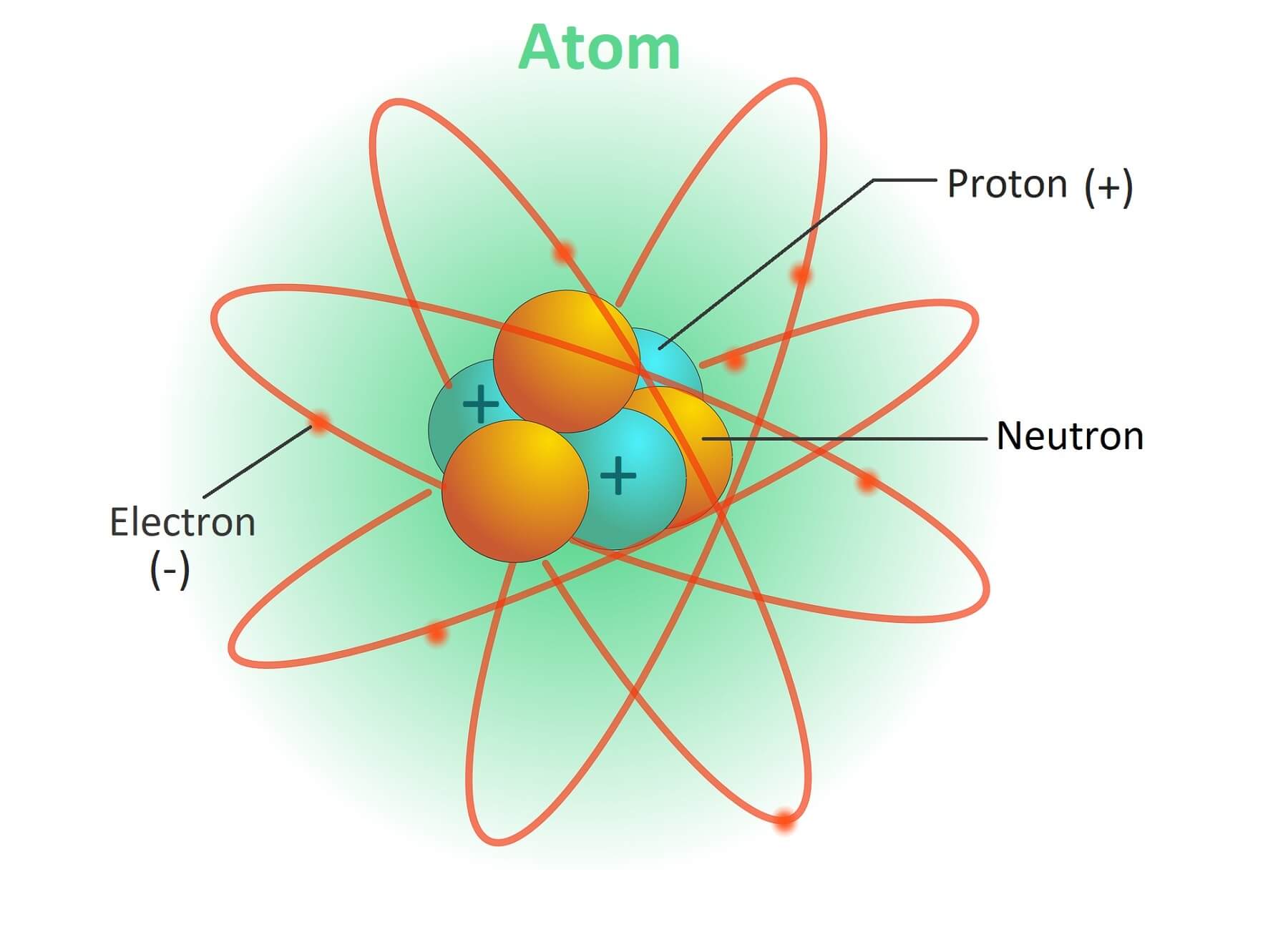
- Atoms are made up of three types of particle: proton, neutron and electron.
- An atom consists of a central nucleus with protons and neutrons. Protons have a positive charge and neutrons have no charge. Hence the nucleus has a positive charge.
- Protons and neutrons are collectively referred to as nucleons.
- The electrons are negatively charged and are located around the nucleus.
- An atom has as many negative electron charges in its nucleus as there are positive protons. Therefore, atoms are electrically neutral.
- Electrons are distributed on a definite track around nucleus and called shells or orbits.
- This concept is given by Bohr. According to Bohr, different electrons revolve around nucleus in their own orbit.
- Atom has been compared to the solar system. In an atom, nucleus is the sun and electrons are planets like planets in the solar system. Each electron rotates around the nucleus in its own orbit. Orbit shell is represented by letter K, L, M, N etc.
- The maximum number of electrons in the shell provided by the 2n2 formula where n is the number of energy shell orbits. K [Shell] – 2 × 12 = 2 k shell can have two electrons.
- Electrons, which are present in the outermost atomic shell are called valence electrons. And the shell that occupies valence electrons is called valence shell.
Electron
- An electron is a subatomic particle with a negative charge.
- They are free (not attached to an atom) or attached to the nucleus of an atom.
- The electrons in the atoms are in spherical shells with different radii that indicate energy levels.
- The larger the spherical shell, the more electron energy will be.
- In electrical conductors, current flows from electrons, moving one at a time from one atom to another atom, and generally from negative to positive electrodes.
- In semiconductor materials, electricity also occurs when electrons are moving. In some cases, however, it is easier to understand current as electron-poor motion from atom to atom. An atom without electrons in a semiconductor is called a hole. Cavities generally “move” from positive to negative electrodes.
- The charge on an electron is seen as the unit of electrical charge, Negative polarity is set.
- The electron charge is the same but inversely proportional to the positive charge on the proton or hole.
- Electric charge is usually not measured by the charge of a single electron because the charge is very small. Instead, the standard unit is the amount of electrical charge in Coulomb, which is denoted by C and represents about 6.24 x 10-18 electrons.
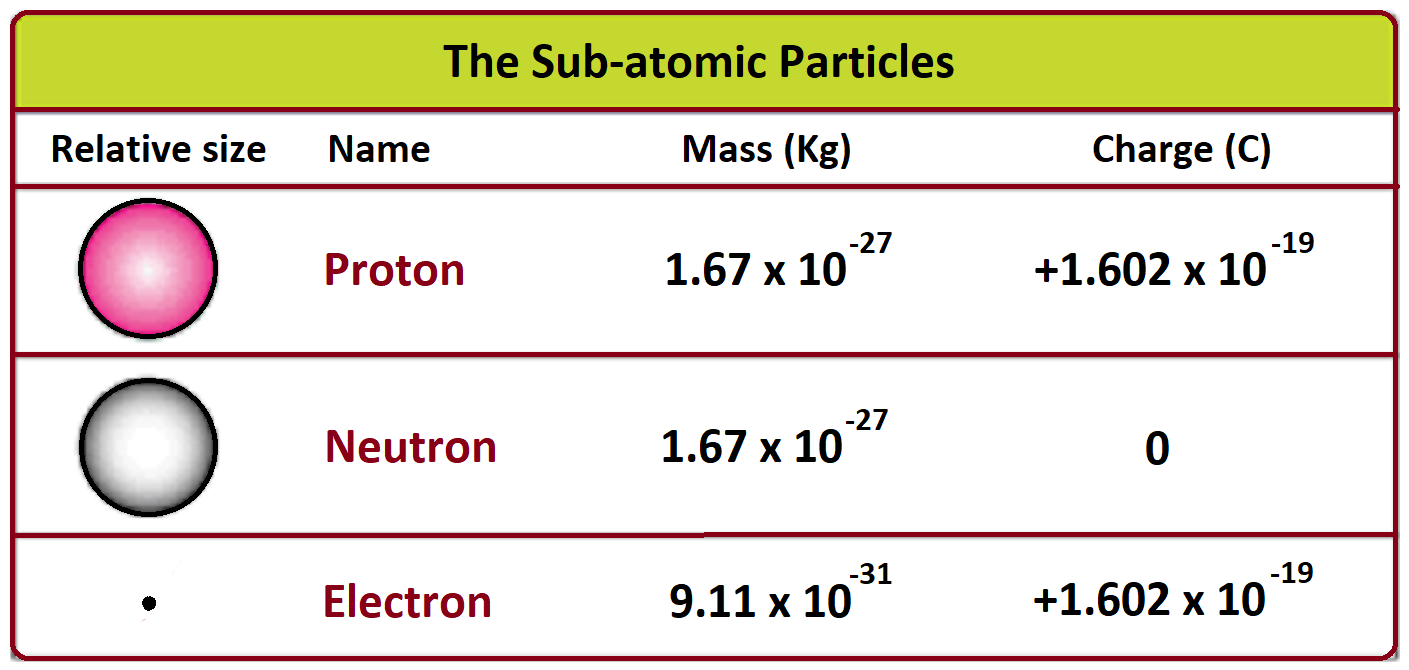
- The electron charge denoted by “e” is about 1.60 x 10-19 C.
- The mass of the electron at rest is about 9.11 x 10-31 kg (kg).
Proton
- Protons are positively charged particles that are located in the nucleus of an atom.
- It was discovered by Rutherford during experiments with cathode ray tubes between 1911 and 1919.
- The mass of the protons is about 99.86, and the neutron also has same mass.
- The number of protons in an atom is unique for each element. For example, carbon atoms have six protons, hydrogen atoms have one, and oxygen atoms have eight protons.
- The number of protons in an atom is determined by the atomic number of this element.
- The number of protons also determines the chemical behavior of the element.
- The elements of the periodic table are arranged in order of increasing atomic number.
Each proton consists of three quarks – two “high” quarks (each with two thirds positive charge) and one “low” quark (one third negative) – and other subatomic particles, the gluons, which are massless and held together.
Neutron
- The existence of neutrons was explained by Rutherford in 1920 and discovered by Chadwick in 1932, according to them Neutrons were found during the experiment when atoms were shot onto a thin layer of beryllium.
- An uncharged subatomic particle was released – a neutron.
- Neutrons are no-charge particles that occur in all atomic nuclei (except hydrogen).
- The mass of a neutron is slightly larger than the mass of a proton.
Neutrons, like protons, are made up of quarks – a “high” quark (with a positive charge of 2.3) and two “low” quarks (each with a negative third charge.
What are the Molecules?
A molecule is generally a group of two or more atoms that are chemically bound together. Atoms join chemically with external power such as heat and light, this leads to molecular formation. During the formation of molecular atoms lose valence electrons or get electrons from other atoms.
For example; If two wax sheets must join, they are heated. After heating the two pieces of pressed together to form a structure. Here it is a chemical factor that is responsible for joining the molecule of the candle pieces
There are two main types of bonds: 1. Covalent Bond 2. Ionic Bond
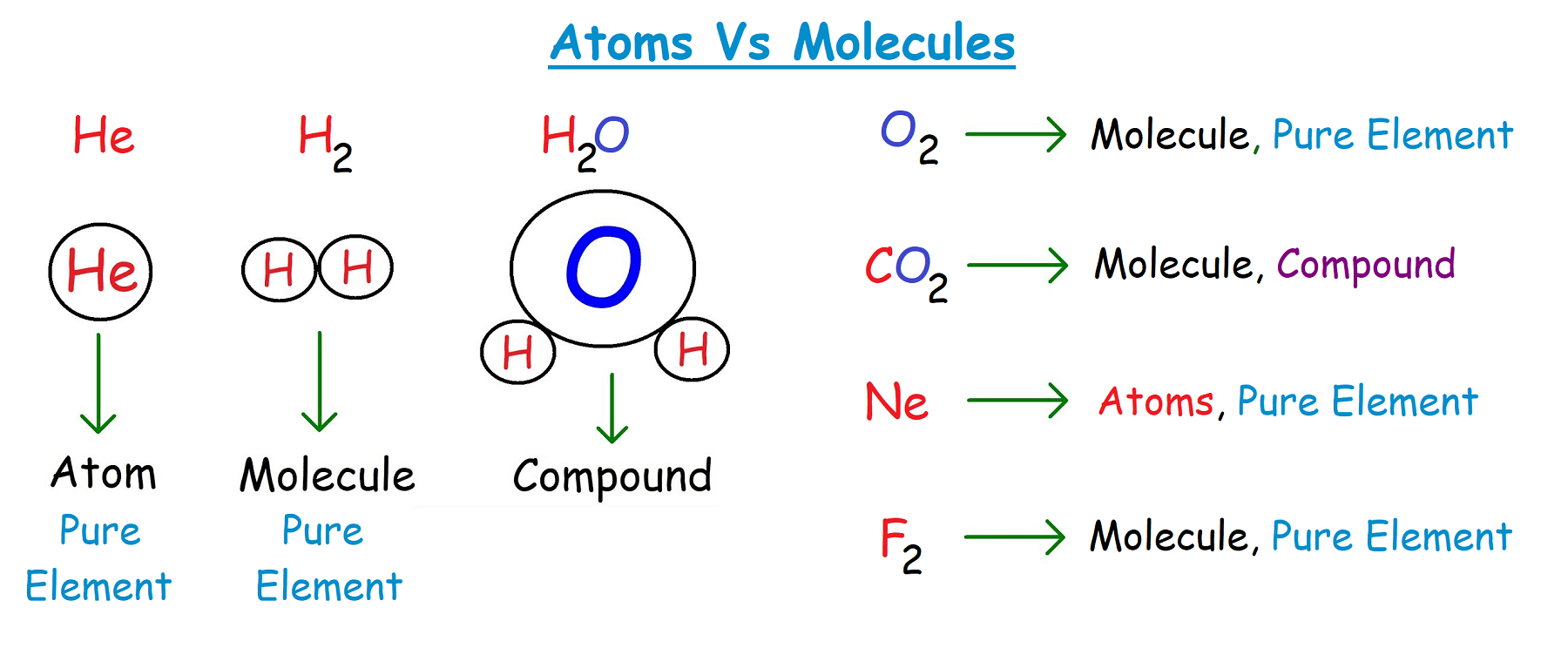
Homoatomic molecules: If molecules consist of the same atom of the same element called the homo atomic molecules, Like Hydrogen (H2), Nitrogen (N2), Oxygen (O2) etc…
Heteroatomic molecule: if a molecule is made up of different atoms of the different elements it is called the heteroatomic molecule, Like Water (H2O), Methane (CH4) etc…
Difference in between Atoms and Molecules:
- From a scientific point of view, an atom is the smallest unit of an element that may or may not freely exist. On the other hand, a molecule is a group of atoms held together by a bond and is the smallest unit in a compound.
- In addition, an atom may or may not be free. On the other hand, molecules exist in the free state. In addition, an atom consists of a nucleus (made up of neutrons and protons) and an electron. Conversely, a molecule consists of two or more atoms of the same or different atoms that are chemically linked.
- The shape of the atom is spherical, While the shape of the molecule can be angular, rectangular, triangular or linear.
- Atoms are highly reactive and take part in chemical reactions without chemical decomposition. On the other hand, a molecule is less reactive and does not take part in any chemical reactions.
- Atoms maintain the nuclear bond as they involve electrostatic adsorption between the electron and the nucleus. Instead, molecules have covalent bonds, which means they share electrons with each other in order to stay connected.
How many Molecules are Present in an Atom?
Chemists generally use moles as the unit of number of atoms or molecules in a substance. One mole (abbreviated to mol) corresponds to 6.022 × 1023 (Avogadro’s number) and each element has a different molar mass depending on its weight of (6.022 × 1023 of its atoms 1 mole).
John Dalton Atomic Theory
A chemical combination theory, was first stated by John Dalton in 1803. It involves the following postulates:
(1) the element consists of inseparable small particles (atoms).
(2) All atoms of the same element are identical and Different elements have various types of atoms.
(3) Atoms Neither be made nor destroyed.
(4) ‘element of compound’ is formed when the atomic element atom joins the simple ratio to form an ‘atom of compound’ molecule). Dalton also proposes a symbol for different elements.
Examples of atoms and molecules
|
Atoms |
Molecules |
|
| Examples | Oxygen (O) | Water (H2O) |
| Hydrogen (H) | Sodium chloride (NaCl) | |
| Argon (Ar) | Nitrogen (N2) | |
| Plutonium (Pu) | Carbon dioxide (CO2) |