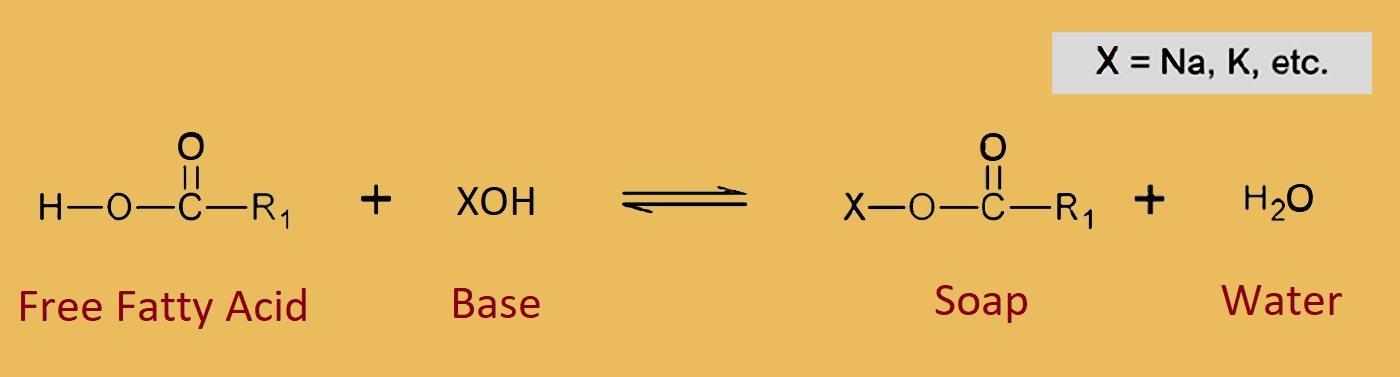Have you ever wondered that why are bubbles formed in soap solution? What’s the science behind it? When you add water in soap solution, you can see those bubbles in the solution. But have you ever thought about it, why does it happen? Let’s study about it in details. First of all, we should know about soap. What are their types, which chemicals are present in it?
CONTENT INDEX
About Soaps
Soaps are sodium or potassium salts in which fatty acids are present in higher amount. Fatty acids are stearic acid, oleic acid, palmitic acid. But how does it form?
How does Soap form?
When we heat sodium or potassium hydroxide (KOH) with fatty acids, then Soap is formed by this reaction. This reaction is called Saponification.
Chemical formula of Soap = C17H35COONa
sodium or potassium soaps are soluble in water that’s why it is used for cleansing purposes.
Types of Soaps
1) Bathing soaps: potassium soaps are soft to the skin, therefore it is used as bathing soaps. They are prepared by using potassium hydroxide (KOH) instead of NaOH.
2) Toilet Soaps: Soap contains a higher amount of total fatty matter (TFM). Higher the TFM, the better cleaning performance of the soap. Higher TFM also means that soap will provide better moisturizing effect.
3) Medicated Soaps: Medicated soaps are those antibiotics that contain natural ingredients. Heals dry skin and makes it smoother than before. Medicated soap has many functions, such as moisturizer, antibiotic, mild fragrance, and can even be used as anti-fungal soap.
4) Shaving Soaps: Shaving soap is a rough soap that is used to Lather with the shaving brush. The foam it produced is used to cover the face during shaving, soften the hair, and prepare for shaving.
5) Laundry Soaps: Although people usually refer to washing powder as “soap“, it is actually a synthetic mixture that acts very similar to soap, but has some major improvements. Soap has a cleansing effect, because each soap molecule is composed of a hydrocarbon chain and a carboxyl group (fatty acid), which have two important functions.
How does Soap Work?
As you know that when you wash your hand, it removes bacteria, but it does not kill the bacteria. Do you know that how does it work? let ‘s study about it.
You know, you should wash your hand at least 20 seconds, because it is the effective way to remove bacteria fully from your hands despite it doesn’t kills them. For killing bacteria, we should use antibacterial soap instead of regular soap. Add a solid base to an unsaturated fat, and permit saponification to yield an ideal substance for cleaning. Various particles in an atom have varying avarice for electrons.
Oxygen is extremely eager and in water, it takes the electron thickness away from the two hydrogens. This outcomes in a polar particle, with one end being more certain and the other more negative. adjoining water atoms would then be able to interface Positive (+) to Negative (-), similar as two bar magnets.
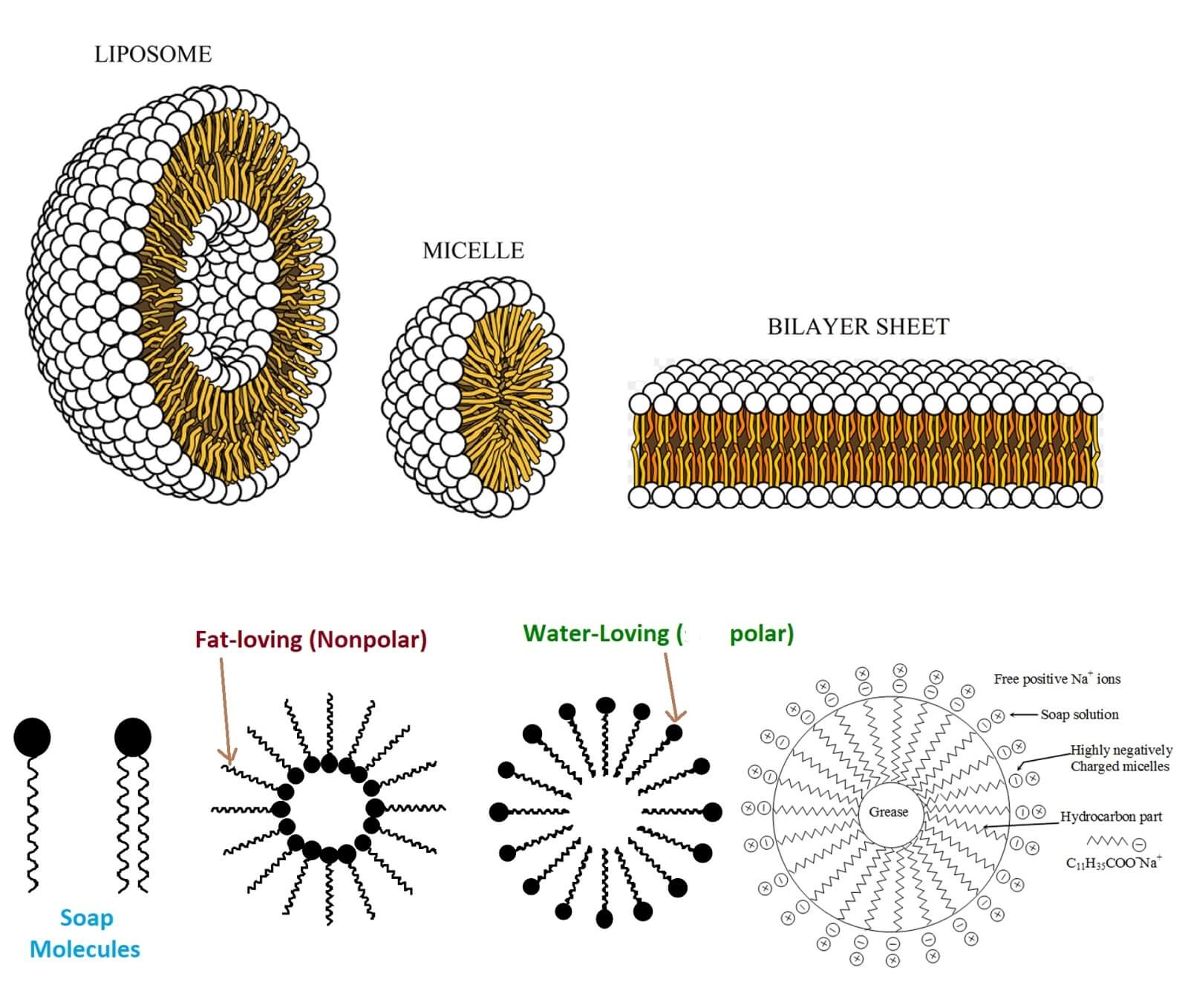
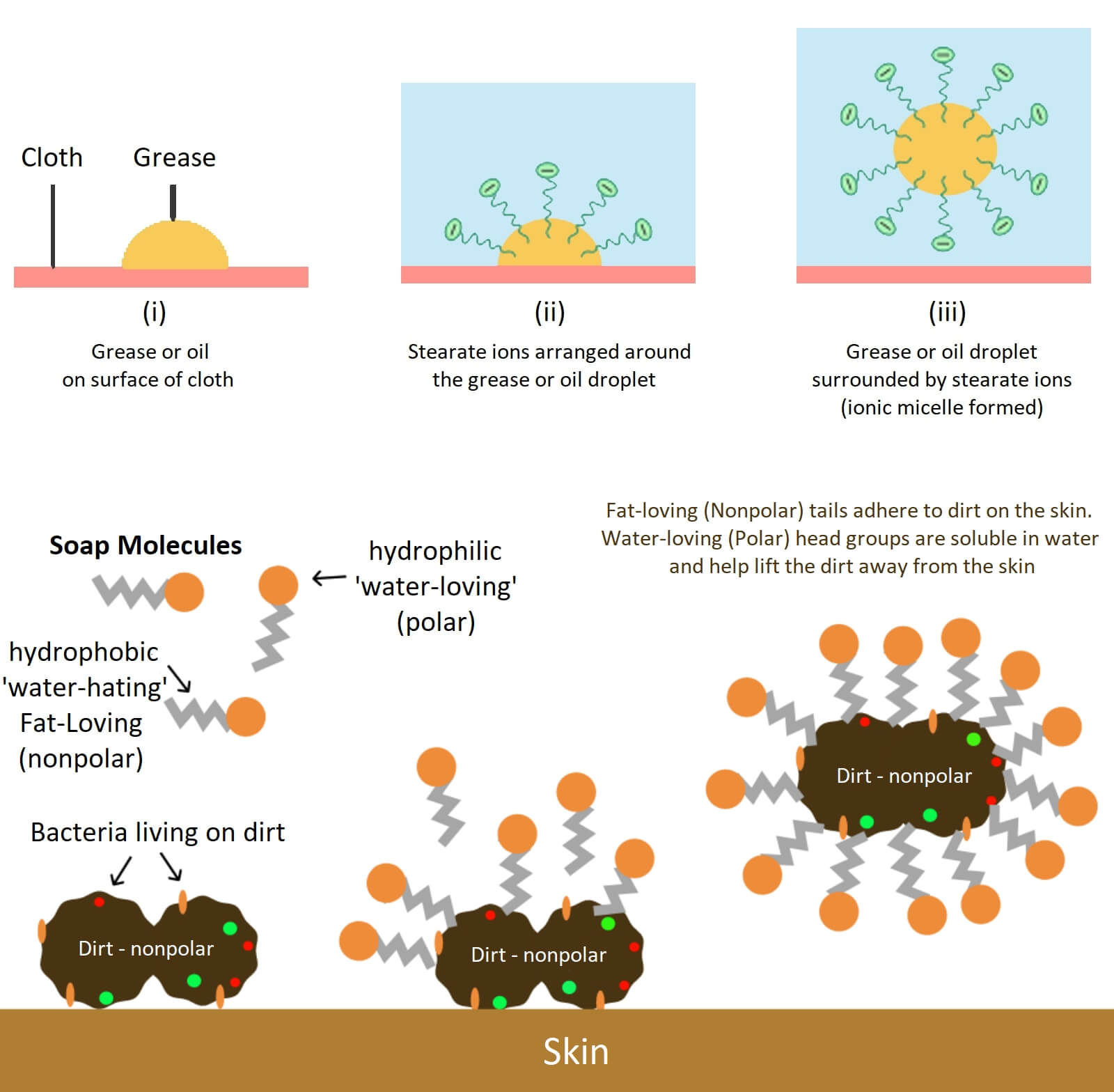
Atoms like hydrocarbons are considered nonpolar or fattier on the grounds that they share their electrons generally similarly, which means their communications with their neighbors are far weaker. It is one of phrases ”Like dissolve like” attempt to blend oil or vinegar and the two will simply isolate out, that is on the grounds that it’s good for polar substances to spend time with other polar substances while nonpolar ones hang out somewhere else. The particles are quite long with one end being polar and the other being nonpolar. In case, If you clean an oily plate, then any polar object will break down directly into the water, while any non-polar object is protected by a circles of cleanser atoms called micelles.
The nonpolar closures of the cleanser trap and warm up to unsaturated fats, while the polar finishes connect with the water, permitting any oil to be washed away. with regards to cleaning your hands, cleanser plays a comparable role. A great many microbes cling to your skin, and their external layers are in reality very cleanser like, with nonpolar greasy pieces in the center and polar parts outwardly.
In some conditions, soap can also break down the bacterial cell membranes and kill them off. Now you probably understand that how does soap work? how its formed? but the answer of one question is still remained that why are bubbles formed in soap solution?
Why are Bubbles Formed in Soap Solution?
Obviously, you know that how to make bubbles it is simple as that just add water in soap solution but how does it form? why we add water in the soap to make bubbles the reason is because of something called the surface tension of water. water molecules would like to stick together and don’t like to come apart. when you add soap to the water, soap allows the water molecules to stretch and expand. so, when you blow air into the bubbles, air goes inside stretches the surface tension of the water and there you go.
Why Soap Bubbles are Round in Shape?
Bubbles are round in shape cause air exerts the same pressure in all directions so that’s why it is form sphere shape.
Why do Bubbles Pop Easily?
Because this is in water now, bubbles have a really tiny amount of water in them. When the water disappears, bubbles will also disappear. But the reason of disappearing of water cause when day is hot. you can see in hot day bubbles don’t last too long. but in rainy days bubbles keep floating for a long time because the air around the bubbles in immediately after the rains is filled with water molecule. It’s very humid at that time so bubbles float. So, when you touch bubbles, little oil is present on your skin so when we touch bubbles so you no longer have soap to stretch the surface tension of the water your bubbles disappear.
Why are Soap Bubbles Colorful?
When you see bubbles, they look colorful. Have you ever noticed that why they look colorful?
We can give answer of this question in two ways because light shows dual nature. First is an electromagnetic wave and second nature of light is particles of light called photons. This is called wave particle duality of light. Now we will give answer of this question why bubbles look so colorful. Assuming that light is a wave if you have ever looked into a lake, you have probably noticed that you can see fish swimming beneath the surface. but if you shift your focus, you can see your reflection that because light partially reflects off a watery surface.
The wall of a soap bubble is just a thin film of water sandwiched between two layers of soap so when light hits the first surface some lights reflected and some light goes through just like the surface of a lake. Sometimes the ray of light that goes through will get reflected off the second surface waves have this property where they can interfere with each other. If they are both in phase that is if their peaks and trough line up, they amplify each other in a process called constructive interference. If they are out of phase that is one of the wave picks line up with the troughs of the other wave. they will cancel out in a process called destructive interference.
White light is made up of all the colors of rainbow and different colors correspond to different wavelengths. which wavelengths amplify and cancel out depends on the thickness of the water. this thickness varies all over the soap bubbles so different wavelengths of light are being cancelled out and amplified a different part of the bubble and that’s why we see different colors in different areas this phenomenon is called iridescent.
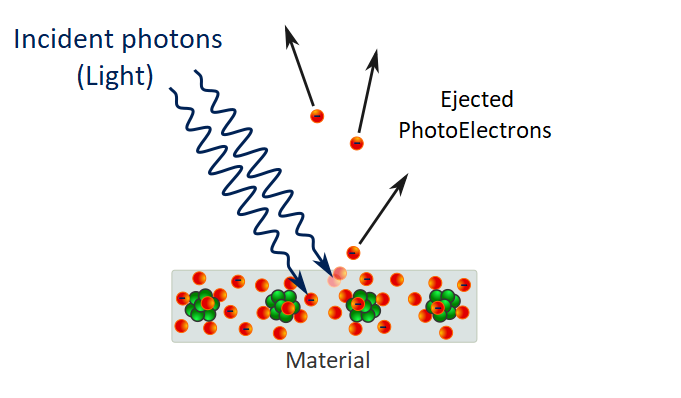
When Light behave like Particles
But it’s half explanation cause light also behave like particle. Particles behave in a similar way. Particle’s don’t interferes with each other so they can’t cancel out or amplify to each other. when we see a glass, we can see our mirror image. It is because of reflection. when light is transmitted through glass photons are reflected. but if the glass is thicker more photons will be reflected. and if glass is thinner no photons will be reflected. So, it depends on the thickness of glass. But maximum 16 photons can be reflected.
After that the graph will be down and this cycle will be repeat again and again. So, 4 photons reflected from each surface and 8 photons will be reflect from two surfaces. Now the question is why bubbles looks colorful. When sunlight which contains all rainbow colors shines on soap bubble the areas that strongly reflect each of these colors overlap and produce all kinds of combination which we see as different colors.
Poonam chhapola