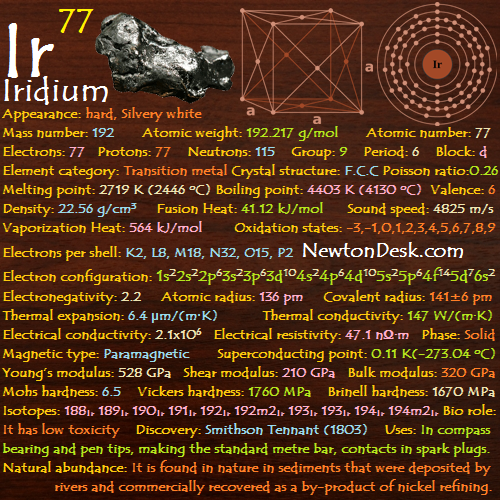
50 Sn (Tin)

Tin is soft, malleable, silvery-white metal, has a highly crystalline structure.
It is not easily oxidized, it’s resists to corrosion, and can be attacked by strong acids, alkalis and acid salts.
Ordinary tin is composed of 9 stable isotopes and 18 unstable isotopes are also known.


Identity
CAS Number: CAS7440-31-5
CID Number: CID5352426
RTECS Number: RTECSXP7320000
CONTENT INDEX
Basic Properties of Tin
Appearance: Silvery-white (beta-β) above 13.2oC or gray (alpha-α) below 13.2oC
Allotropes: Alpha-α, Beta-β
Mass Number: 119
Standard Atomic weight: 118.710 g/mol
Atomic number (Z): 50
Electrons: 50
Protons: 50
Neutrons: 69
Period: 5
Group: 14
Block: p
Element category: Post-transition metal
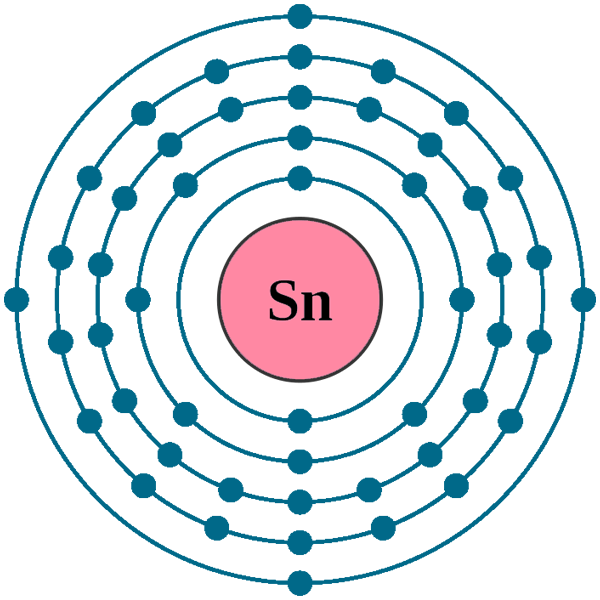
Electrons per shell: K2, L8, M18, N18, O4
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p2
Thermal Properties of Tin
Phase: Solid
Melting point: 505.08 K (231.93 oC, 449.47 oF)
Boiling point: 2875 K (2602 oC, 4716 oF)
Debye Temperature: 170 K (-103.15 oC, -153.67 oF)
Fusion heat: β: 7.03 kJ/mol
Vaporization heat: β: 296.1 kJ/mol
Specific heat: 217 J/(kg K)
Molar heat capacity: β: 27.112 J/(mol.K)
Thermal expansion: 22 μm/(m∙K)
Thermal conductivity: 66.8 W/(m∙K)
Electrical properties of Tin
Electrical conductivity: 9.1×106 S/m
A Electrical resistivity: 115 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 3.72 K (-269.43 oC, -425.97 oC)
Magnetic Properties of Tin
A Magnetic type: α: Diamagnetic, β: Paramagnetic
Magnetic susceptibility (xmol): α: +3.1×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000227
Mass magnetic susceptibility: -3.1×10-9 m3/kg
Molar magnetic susceptibility: -0.368×10-9 m3/mol
Physical Properties of Tin
Density: β: 7.265 g/cm3 (In solid), α: 5.769 g/cm3 (In solid), 6.99 g/cm3 (In Liquid)
Molar volume: 0.00001624 m3/mol
Young’s modulus: 50 GPa
Shear modulus: 18 GPa
Mohs Hardness: 1.5
Bulk modulus: 58 GPa
Poisson ratio: 0.36
Brinell hardness: 50-440 MPa
Sound Speed: 2730 m/s
Atomic Properties of Tin
Oxidation states: 4, 3, 2, 1, -1, -2, -3, -4
Valence Electrons: 5s2 5p2
Ion charge: Sn4+ , Sn2+
The ionization potential of an atom: 7.37
Ionization energies: 1st: 708.6 kJ.mol 2nd: 1411.8 kJ/mol 3rd: 2943 kJ/mol
Ionic radius: 69 pm
Van der waals radius: 217 pm
Atomic radius: empirical: 142 pm
Covalent radius: 139±4 pm
Filling Orbital: 5p2
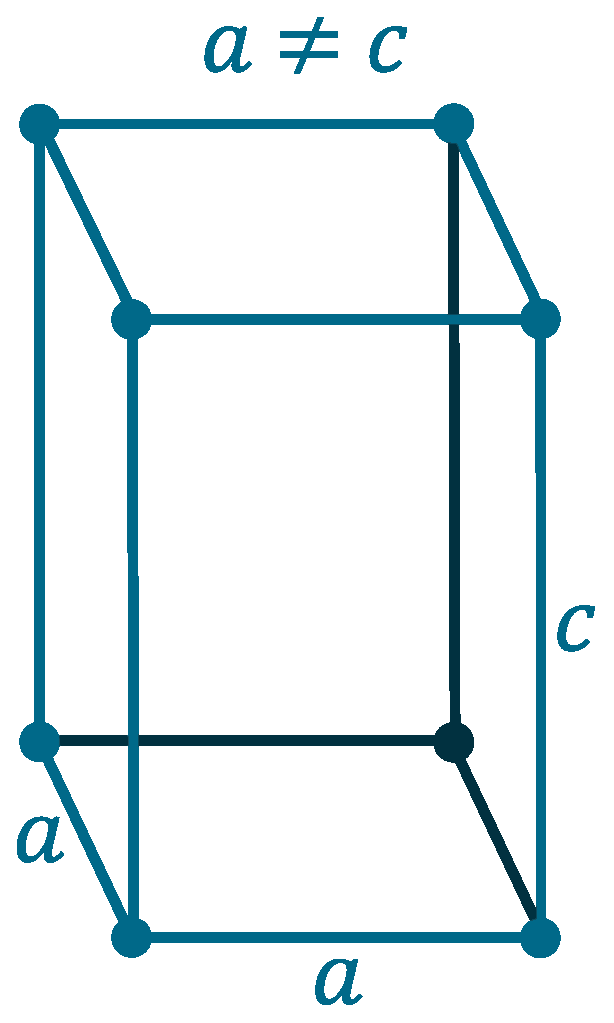
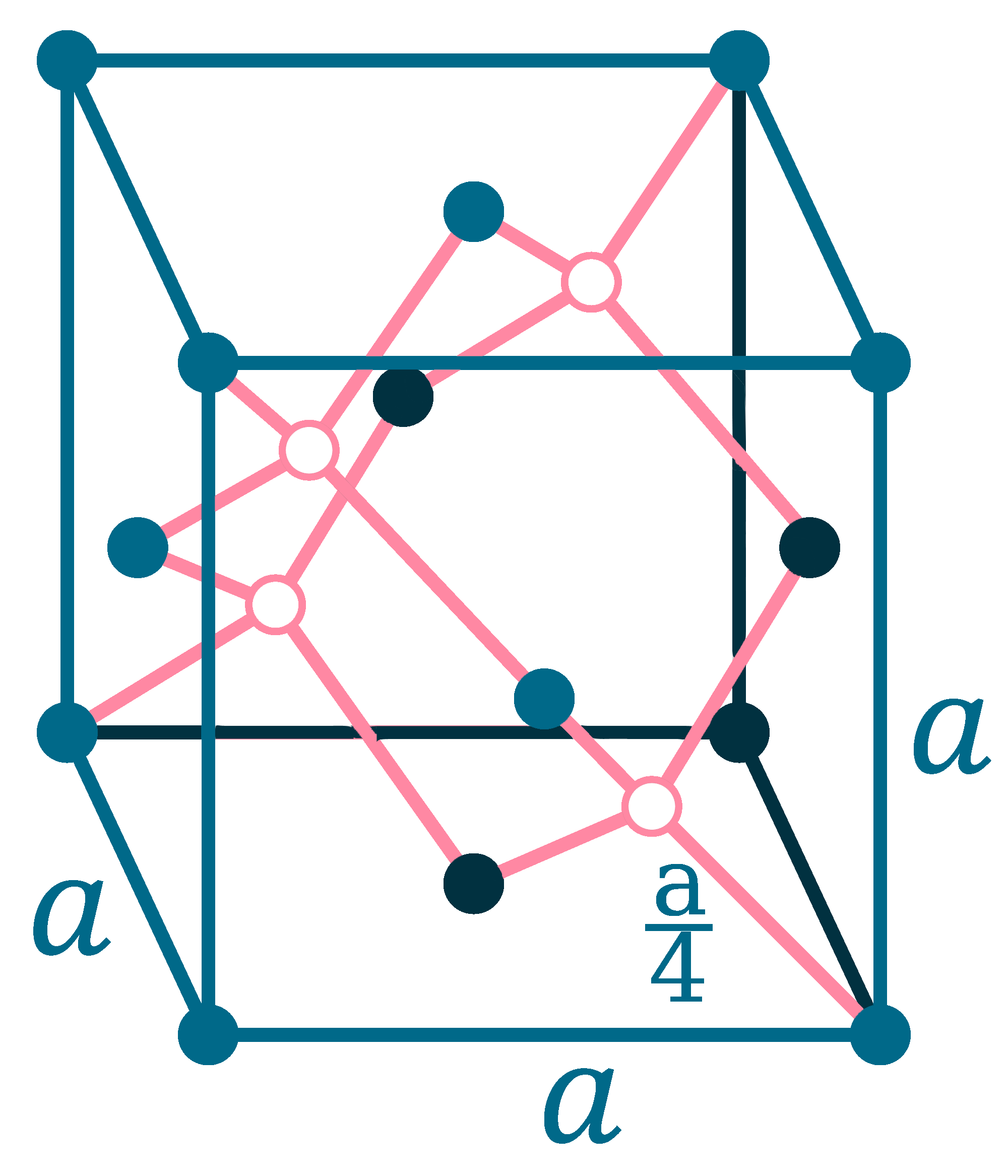
Crystal structure: β: Tetragonal (Above 13.2oC), α: Face-centered diamond cubic (Below 13.2oC)
Lattice angles: π/2, π/2, π/2
Lattice constant: 583.18, 583.18, 3.181 pm
Grid parameters: a=5.831 Å c=3.181 Å (Tetragonal)
Attitude c/a: 0.546
Space Group Name: I41/amd
Space Group Number: 141
Reactivity of Tin
Electronegativity: pauling scale: 1.96
Valence: +4
Electron affinity: 107.3 kJ/mol
Nuclear Properties of Tin
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3P0
Neutron cross section (Brans): 0.62
Neutron Mass Absorption: 0.0002
Isotopes: 112Sn 114Sn 115Sn 116Sn 117Sn 118Sn 119Sn 120Sn 122Sn 124Sn 126Sn
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 112Sn | 0.97 | 11.905 | Stable |
| 114Sn | 0.66 | 113.903 | Stable |
| 115Sn | 0.34 | 114.903 | Stable |
| 116Sn | 14.54 | 115.902 | Stable |
| 117Sn | 7.68 | 116.903 | Stable |
| 118Sn | 24.22 | 117.902 | Stable |
| 119Sn | 8.59 | 118.903 | Stable |
| 120Sn | 32.58 | 119.902 | Stable |
| 122Sn | 4.63 | 121.903 | Stable |
| 124Sn | 4.63 | 123.905 | Stable |
| 126Sn | Tace | – | 2.3×105 y |
Chemical Reactions
On heating, It reacts with oxygen and form Tin dioxide: (But stable at ambient temperature)
Sn (s) + O2 (g) → SnO2 (s)
Stable with water at ambient condition, but React with hot water and form Tin dioxide and hydrogen:
Sn (s) + 2H2O (g) → SnO2 (s) + 2 H2 (g)
Sn(IV) is precipitated as α-tin acid upon hydrolysis of Sn(IV) solutions:
[SnCl6]2- (aq) + 6 H2O (l) ⇌ H2[Sn(OH)6] (s) + 4 H+ (aq) + 6 Cl– (aq)
The precipitate in amphoteric and is dissolved in acids and strong alkali:
H2[Sn(OH)6] (s) + 2 OH– (aq) ⇌ [Sn(OH)6]2- (aq) + 2 H2O (l)
H2[Sn(OH)6] (s) + 4 H+ (aq) + 6 Cl– (aq) ⇌ [SnCl6]2- (aq) + 6 H2O (l)
Reacts with Chlorine and form Tin (IV) chloride:
Sn (s) + 2 Cl2 (g) → SnCl4 (s)
Tetraalkyl and tetraaryltin compounds can be prepared using Grignard reagents:
SnCl4 + 4 RMgBr → R4Sn + 4 MgBrCl
The mixed halide-alkyls, are prepared by redistribution reactions (R represent substituents or hydrogen atoms):
SnCl4 + R4Sn → 2 SnCl2R2 (R2Sn, as seen for singlet carbenes)
Tin History
Naming: Symbol Sn from Latin: stannum (tin). Tin from Anglo-Saxon
Discovery: Around 3500 BC
Tin Uses
It is used to coat other metals to prevent corrosion, such as in tin cans (made of tin-coated steel), tin-plated steel containers are widely used for food preservation.
Alloys of Tin are employed in many ways, such as soft solder (joining pipes or electric circuits), pewter, bell metal, babbitt metal (89% Tin, 9% Antimony and 2% Copper), dental amalgams (50% mercury, ~22–32% silver, ~14% tin, ~8% copper and other trace metals), bronze and phosphor bronze.
A niobium-tin alloy is used for superconducting magnets.
Tin oxide is used for ceramics and in gas sensors.
Tin salts sprayed onto glass, which is used to produce electrically conductive coatings.
Indium and Tin oxides are electrically conductive and transparent, so It is used in Optoelectronics devices such as Liquid crystal displays (LCD).
Ttin(II) chloride is used as a reducing agent and as a mordant for dyeing calico and silk.
Zinc stannate (Zn2SnO4) is a fire-retardant used in plastics.
Biological role
The metal is non-toxic, but some organo-tin compounds are highly poisonous as toxic as cyanide and it should be handled with care.
The effect of organic tin compounds can vary.
The small amount of tin found in canned foods is quite harmless even the limit of tin content in U.S. foods is 300 mg/kg.
Humans can absorb tin compounds through food, breathing and the skin.
Uptake of tin compounds can cause acute and long term effects, such as eye and skin irritations, breathlessness, headaches, depressions, liver damage, brain damage etc..
Abundance of Tin
Tin is found chiefly in cassiterite (tin (IV) oxide, SnO2) mineral.
Tin is obtained by reducing the ore with coal in a reverberatory furnace or electric furnace.
Annual world wide production is around 2,53,000 tons.
4×10-7% (In Universe)
12×10-5% (In Meteorites)
9×10-7% (In Sun)
0.00022% (In Earth’s Crust)
9.9×10-10% (In Oceans)
0.00002% (In Humans)

World’s Top 3 producers of Tin
1) China
2) Indonesia
3) Peru
World’s Top 3 Reserve holders of Tin
1) China
2) Indonesia
3) Brazil
#tin