21 Sc (Scandium Element)

Scandium is a soft, silver transition metal, which develops a slightly yellowish or pinkish cast upon exposure to air.
It tarnish in air, where it reacts and readily forms a white coating of nitride, and when once ignite it burn easily with a yellow-red flame.
It reacts with water to form hydrogen gas and will dissolve in many acids, but It is not attacked by a 1:1 mixture of HNO3 and 48% HF.


Identity
CAS Number: CAS7440-20-2
CID Number: CID23952
DOT Hazard Class: 4.1
DOT Number: 3089
CONTENT INDEX
Basic Properties of Scandium
Pronunciation: Skan-dee-am
Appearance: Silvery white
Mass Number: 45
Standard Atomic weight: 44.955 g/mol
Atomic number (Z): 21
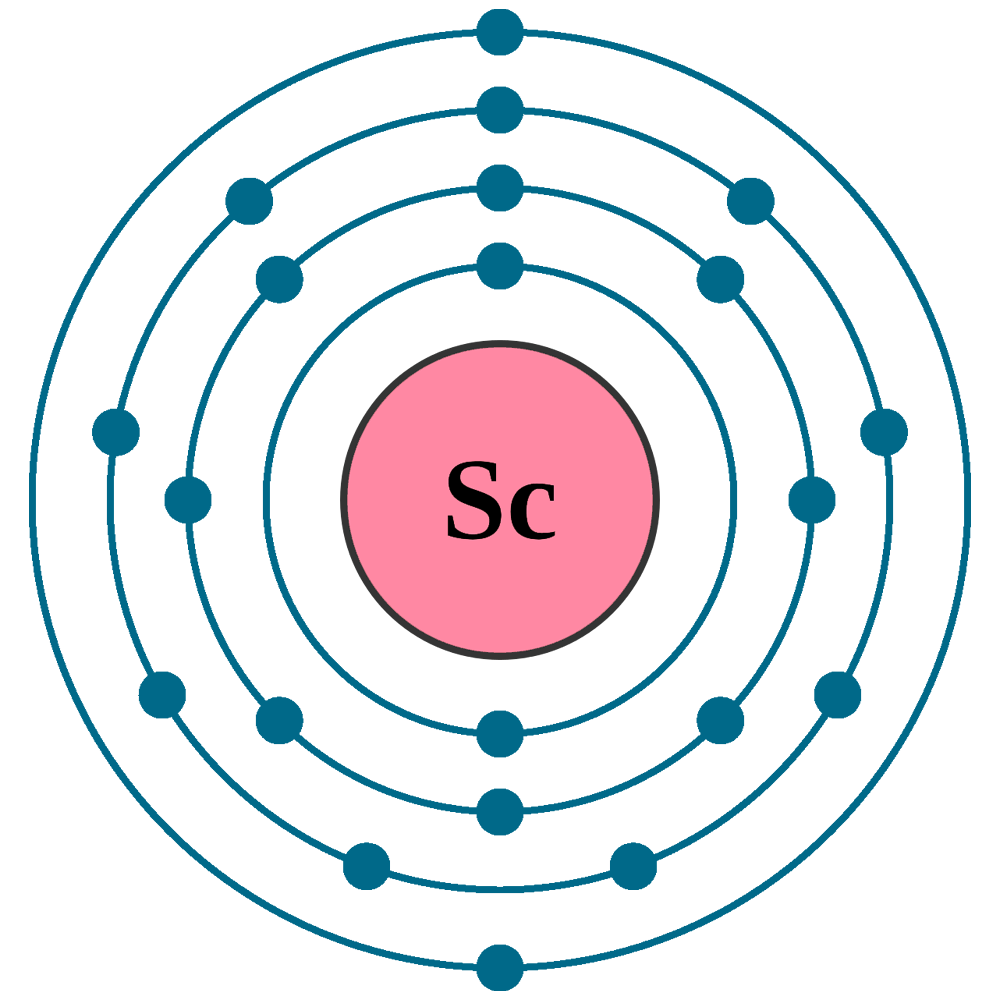
Electrons: 21
Protons: 21
Neutrons: 24
Period: 4
Group: 3
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M9, N2
Electron configuration: 1s22s22p63s23p63d14s2
Thermal Properties of Scandium
Phase: Solid
Melting point: 1814 K (1541 oC, 2806 oF)
Boiling point: 3109 K (2836 oC, 5136 oF)
Fusion heat: 14.1 kJ/mol
Vaporization heat: 332.7 kJ/mol
Specific heat: 567 J/(kg K)
Molar heat capacity: 25.52 J/(mol.K)
Thermal expansion: 10.2 μm/(m∙K) (α-poly)
Thermal conductivity: 15.8 W/(m∙K)
Electrical properties of Scandium
Electrical conductivity: 1.8×106 S/m
A Electrical resistivity: 562 nΩ∙m (α-poly)
A Electrical type: Conductor
Critical point (Superconducting point): 0.5 K (-273.1 oC, 459.58 oF)
Magnetic Properties of Scandium
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +315×10-6 cm3/mol
Volume magnetic susceptibility: 0.0002627
Mass magnetic susceptibility: 88×10-9 m3/kg
Molar magnetic susceptibility: 3.956×10-9 m3/mol
Physical Properties of Scandium
Density: 2.985 g/cm3 (In solid) 2.80 g/cm3 (In Liquid at M.P)
Molar volume: 0.000015061 m3/mol
Young’s modulus: 74.5 GPa
Shear modulus: 29 GPa
Bulk modulus: 56.6 GPa
Poisson ratio: 0.279
Brinell hardness: 736-1200 MPa
Atomic Properties of Scandium
Oxidation states: 3, 2, 1
Valence Electrons: 3d1 4s2
Ion charge: Sc3+
Ionization potential of an atom: 6.57
Ionization energies: 1st: 633 kJ.mol 2nd: 1235 kJ/mol 3rd: 2389 kJ/mol
Ionic radius: 74.5 pm
Atomic radius: 162 pm (empirical)
Van der Waals: 211 Pm
Covalent radius: 170±7 pm
Filling Orbital: 3d1
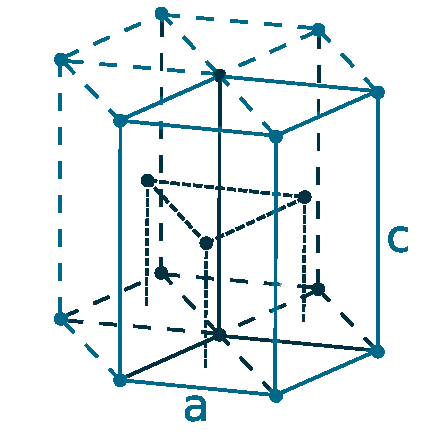
Crystal structure: Hexagonal close packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 330.9, 330.9, 526.8 pm
Grid parameters: a=3.309 Å c=5.268 Å
Attitude c/a: 1.592
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Scandium
Electronegativity: 1.36 (pauling scale)
Valence: +3
Electron affinity: 50 kJ/mol
Nuclear Properties of Scandium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2D3/2
Neutron cross section (Brans): 27.2
Neutron Mass Absorption: 0.025
Isotopes: 44m2Sc 45Sc 46Sc 47Sc 48Sc
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 44m2Sc | Syn | – | 58.61 h |
| 45Sc | 100 | 44.956 | Stable |
| 46Sc | Syn | – | 83.79 d |
| 47Sc | Syn | – | 80.38 |
| 48Sc | Syn | – | 43.67 h |
Chemical Reactions of Scandium
The metal tarnishes in air and burns readily, and forms scandium (III) oxide:
4 Sc + 3 O2 → 2 Sc2O3
When finely divided (powdered form) or heated, It dissolves in water, and forming Sc(III) hydroxide and hydrogen gas.
2 Sc (s) + 6 H2O (l) → 2 Sc(OH)3 (aq) + 3 H2 (g)
Reacts with halogens, and forming Sc (III) halides:
2 Sc (s) + 3 F2 (g) → 2 ScF3 (s) (Scandium (III) fluoride)
2 Sc (s) + 3 Cl2 (g) → 2 ScCl3 (s) (Scandium (III) chloride)
2 Sc (s) + 3 Br2 (g) → 2 ScBr3 (s) (Scandium (III) bromide)
2 Sc (s) + 3 I2 (g) → 2 ScI3 (s) (Scandium (III) iodide)
It dissolves readily in dilute hydrochloric acid (HCL), and forming Sc(III) ions and hydrogen:
2 Sc (s) + 6 HCL (aq) → 2 Sc3+ (aq) + 6 Cl– (aq) + 3 H2 (g)
The Scandium oxide (Sc2O3) and hydroxide (Sc(OH)6) are amphoteric:
Sc(OH)3 + 3 OH− → [Sc(OH)6]3− (scandate ion)
Sc(OH)3 + 3 H+ + 3 H2O → [Sc(H2O)6]3+
Scandium History
Naming: After Scandinavia
Prediction: Dmitri Mendeleev
Discovery and first isolation: Lars Fredrik Nilson (1879)
Scandium Uses
Scandium is one of the rare element , and it is mainly used for research purposes.
It has great potential (having or showing the capacity) because it has almost as low a density as aluminium and a much higher melting point.
Aluminium-scandium alloys are used in the aerospace industry and for sports equipment (high-end bicycle frames, baseball bats, etc.) which rely (depend on with full trust) on high performance materials.
Scandium iodide (I3Sc) is added to mercury vapour lamps to produce a highly efficient light source resembling (look or seem like) sunlight, as like helps in colour televisions to reproduce colour well when filming indoors or night-time.
The radioactive isotope scandium-46 is used as a tracing agent in oil refining to monitor the movement of various fractions, It can also be used to detect leaks in underground pipes.
Uses of scandium are still growing, because of it is suited to produce catalyzers and to polish glass.
Biological role of Scandium
It is not toxic.
But there have been suggestions that some of its compounds might be cancerogenic, therefore it should be handled with care.
Only trace amounts of element reach the food chain, so the average humans daily intake is less than 0.1 mg (microgram).
Abundance of Scandium
The metal is widely distributed on earth, it occurs in very minute quantities in over 800 mineral species.
Scandium is the main component of the very rare mineral thortveitite ((Sc,Y)2Si2O7), which is found in Malagasy and Scandinavia.
It is also found in the residues (a small amount of something that remains after the main part has gone) remaining after the extraction of tungsten from Zinnwald wolframite ((Mn,Fe)WO4), and in wiikite and bazzite (Be3Sc2Si6O18).
It is extracted as a by-product from uranium mill tailings (the residue of something).
Metallic scandium can be prepared by electrolyzed a eutectic melt of potassium, lithium, and scandium chlorides at 700 to 800°C, and Pure scandium is produced by reducing scandium fluoride (ScF3) with calcium metal.
Annual world wide production is around 10 tons.
3×10-6% (In Universe)
0.00064% (In Meteorites)
4×10-6% (In Sun)
0.0026% (In Earth’s Crust)
1.5×10-10% (In Oceans)

World’s Top 3 producers of Scandium
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders of Scandium
1) China
2) CIS Countries (inc. Russia)
3) USA
Scandium Price: Pure (99.995%) metal price is around $1150-$1250 per KG (KiloGram)
#Scandium



For the brainwashed people;
Be not really careful what you think; anger headaches psychosis e.t.c can happen
For the gifted individuals;
Be careful what you think; the thougths may become real with no chance of return.