08 O (Oxygen Element)

CONTENT INDEX
About Oxygen Element
Oxygen is odorless, colorless, and tasteless gas.
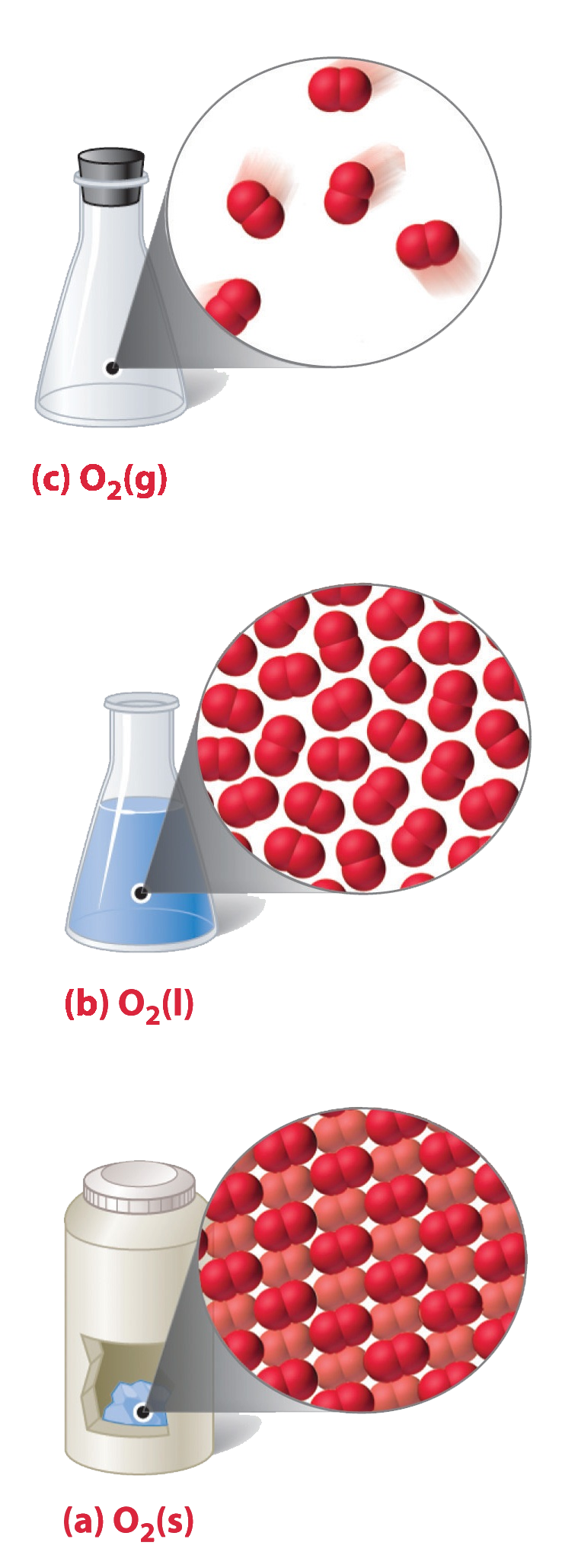
The solid and liquid forms of element are a pale blue color and are strongly paramagnetic.
It is the essential element in the respiratory processes of most of the living cells and as well as in combustion processes.
Ozone (O3) is a highly active compound, which is formed by the action of an electrical discharge or ultraviolet light on oxygen.
Ozone is presence in the atmosphere (amounting to the equivalent of a layer 3 mm thick under ordinary pressures and temperatures) as a shield layer of earth, which helps to prevent from harmful ultraviolet rays comes from the sun to the earth’s surface.
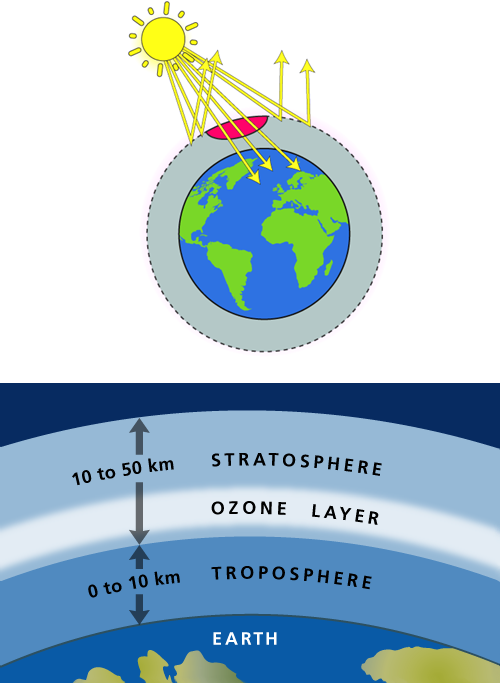
This ozone layer is effecting by the Pollution in the atmosphere.
O3 is toxic and exposure should not exceed 0.2 mg/m.
Color of Undiluted ozone is bluish, Liquid ozone is bluish black and Solid ozone is violet-black.
The oxygen found in the sun plays a part in the carbon-nitrogen cycle (the process once thought to give the sun and stars their energy).
Oxygen under excited conditions is responsible for the yellow-green and bright red colors of the Aurora Borealis (Northern lights).
O2 is reactive and will form oxides with all other elements except noble gases.
Identity
CAS Number: CAS7782-44-7
CID Number: CID977
DOT Hazard Class: 2.2
DOT Number: 1073
RTECS Number: RTECSRS2060000
Basic Properties of Oxygen
Pronunciation: Ok-si-jen
Appearance: Colorless (gas), Pale blue (Liquid and solid)
Allotropes: O2 (Oxygen), O3 (Ozone)
Mass Number: 16
Standard Atomic weight: 15.999 g/mol
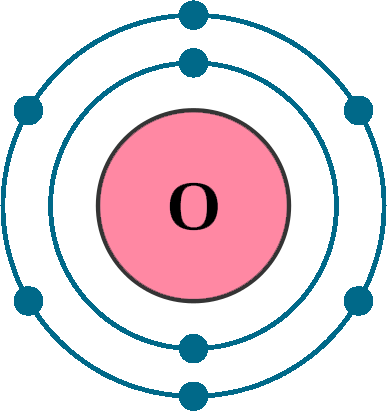
Atomic number (Z): 8
Electrons: 8
Protons: 8
Neutrons: 8
Period: 2
Group: 16
Block: p
Element category: Reactive nonmetal
Electrons per shell: K2, L6
Electron configuration: 1s22s22p4
Thermal Properties of Oxygen
Phase: Gas
Melting point: 54.36 K (-218.8 oC, -361.84 oF)
Boiling point: 90.185 K (-182.965 oC, -297.337 oF)
Debye temperature: 155 K (-118.15 oC, -180.67 oF)
Triple point Temperature: 54.361 K (-218.789 oC, -361.8202 oF)
Triple point Pressure: 0.1463 kPa (0.00144 Atm)
Critical point Temperature: 154.581 K (-118.569 oC, -181.4242 oF)
Critical point Pressure: 5.043 MPa (49.77 Atm)
Fusion heat: 0.444 kJ/mol
Vaporization heat: 6.82 kJ/mol
Specific heat: 919 J/(kg K)
Molar heat capacity: 29.378 J/(mol.K)
Thermal conductivity: 0.02658 W/(m∙K)
Magnetic Properties of Oxygen
Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +3449×10-6 cm3/mol
Volume magnetic susceptibility: 0.00000190772
Mass magnetic susceptibility: 1335×10-9 m3/kg
Molar magnetic susceptibility: 42.71×10-9 m3/mol
Physical Properties of Oxygen
Density: 1.429 g/L (at STP) 1.141 g/cm3 (In Liquid at B.P)
Molar volume: 0.011196 m3/mol
Sound Speed: 330 m/s (gas)
Atomic Properties of Oxygen
Oxidation states: -2, -1, +1, +2
Valence Electrons: 2s2 2p4
Ion charge: O2-
The ionization potential of an atom: 13.56 eV
Ionization energies: 1st: 1313.8 kJ.mol 2nd: 3388.2 kJ/mol 3rd: 5300.5 kJ/mol
Ionic radius: 140 pm (1.4 Å)
Atomic radius: 48 pm (empirical)
Van der Waals: 152 Pm
Covalent radius: 66±2 pm
Filling Orbital: 4f6
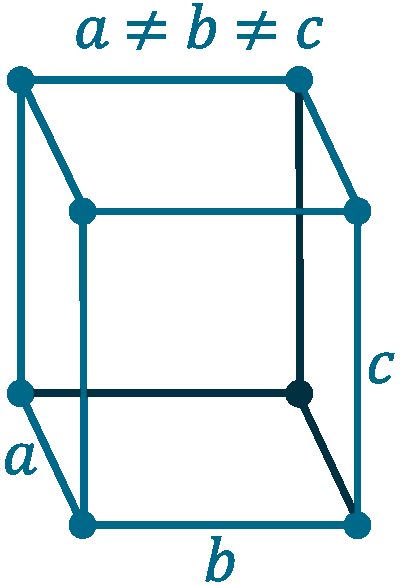
Crystal structure: Cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 540.3, 342.9, 508.6 pm
Grid parameters: a=5.403 Å, b=3.429 Å, c=5.086 Å, β=135.53
Attitude c/a:
Space Group Name: C12/m1
Space Group Number: 12
Reactivity of Oxygen
Electronegativity: 3.44 (pauling scale)
Valence: -2
Electron affinity: 141 kJ/mol
Nuclear Properties of Oxygen
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3P2
Neutron cross section (Brans): 0.00028
Neutron Mass Absorption: 1×10-6
Isotopes: 16O 17O 18O
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 16O | 99.76 | 15.994 | Stable |
| 17O | 0.04 | 16.998 | Stable |
| 18O | 0.20 | 17.999 | Stable |
Chemical Reactions of Oxygen
Reaction with Air
(O2) gas does not react with itself or nitrogen under normal circumstances, but if it exposed to Ultra Violet (UV) light or an electric discharge, then Ozone (O3) is formed:
3 O2 (g) → 2 O3 (g)
Reaction with Water
(O2) doesn’t reacts with water under normal conditions, but dissolved in the water (30 g/l (20 OC).
Reaction with Halogens
(O2) can reacts with fluorine and forms dioxygen difluoride, where it requires irradiation of a mixture at Low pressure (10-20 mm Hg) and Low temperature (77 – 90 K).
O2 (g) + F2 → O2F2 (g)
Reaction with Acids
(O2) gas doesn’t reacts with most acids under normal circumstances.
Reaction with Bases
(O2) gas doesn’t reacts with most bases under normal circumstances.
Reaction with Sulfur
(O2) reacts with sulfur and forming Sulfur Dioxide and Trioxide.
S (s) + O2 (g) → SO2 (g)
2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g)
Oxygen History
Naming: Greek: oxys (acid) and genes (generate), together means “Acid forming”
Discovery: Carl Wilhelm Scheele (1771)
Location: Leeds England (Priestley) / Uppsala Sweden (Scheele)
Named By: Antoine Lavoisier (1777)
Oxygen Uses
Plants and animals depends on oxygen for Respiration, even Hospitals frequently prescribe pure oxygen for patients with respiratory ailments as a medical and biological life support.
Oxygen is largely commercial used in melting, refining and manufacture of steel and other metals, and also used in making synthesis gas for Ammonia (NH3) and Methanol (CH3OH), and as well as used for oxy-acetylene welding and cutting of metals.
Large quantities are also used in the manufacture of a wide range of chemicals including Hydrogen peroxide (H2O2) and Nitric acid (HNO3).
It is also used to make Epoxyethane (ethylene oxide, C2H4O), used as antifreeze and to make polyester, and Chloroethene (C2H3Cl), the precursor to PVC (polyvinyl chloride¸ (C2H3Cl)n).
Used in manufacture of chemicals by controlled oxidation, in Cryogenic engine, Rocket propulsion, Mining, production and manufacture of Stone and Glass products.
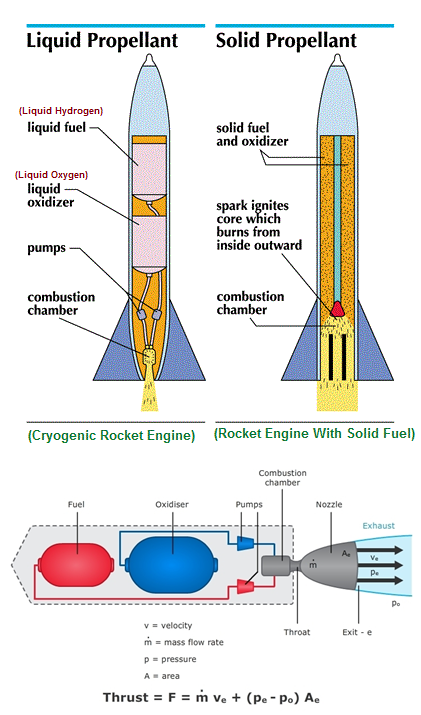
Credit this Image to university of waikato
In Aircraft, An emergency supply of oxygen automatically becomes available for the passenger when the pressure drop suddently.
This oxygen is stored not as an oxygen gas but as the chemical sodium chlorate (NaClO3).
The growing use of oxygen is in the treatment of sewage and of effluent from the industry.
Biological role of Oxygen Element
Around 2 billion (200 crores) years ago, Oxygen first appeared in the Earth’s atmosphere, which accumulates from the photosynthesis of blue-green algae.
Photosynthesis is a process by which energy receives from the sun is used to split water into oxygen (O2) and hydrogen (H), where (O2) passes into the atmosphere and the hydrogen joins with carbon dioxide (CO2) to produce biomass.
6 CO2 + 6 H2O → C6H12O6 + 6 O2
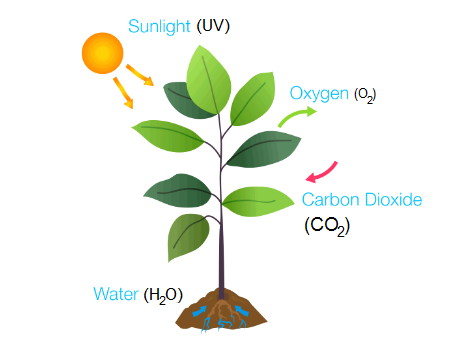
When living things need energy they intake oxygen for Respiration, and returns to the atmosphere in the form of carbon dioxide (CO2).
Anything intake in excess amount could be dangerous for life, similarly
Oxygen is very essential for human being to breathe, but as in so many cases, too much oxygen is not good.
If someone is exposed to large amounts of oxygen for a long time than Lung damage can occur.
Breathing 50 – 100% pure oxygen at normal pressure (Atmospheric pressure) over a prolonged (continuing for a long time) period causes lung damage.
Oxygen is usually stored under very low temperatures, therefore everyone should wear special clothes to prevent the freezing of body tissues.
Highly concentrated sources of oxygen promote rapid combustion, therefore there are fire and explosion hazards in the presence of fuels.
Abundance of Oxygen Element
By volume, 21% oxygen present in the atmosphere, which is is obtained by Liquefaction and Fractional distillation.
In the atmosphere, if volume of oxygen below 17%, then breathing for unacclimatized people becomes difficult, and above 25% many organic compounds are highly flammable.
Earth crust is composed mainly of silicon-oxygen minerals and many other elements as their oxides.
In the Earth’s atmosphere, Oxygens(O2) comes from the photosynthesis of plants.
Oxygen is also fairly soluble in water, which makes life possible in lakes, rivers, and oceans.
Oxygen is the 3rd most abundant element found in the sun.
The atmosphere of Mars contains about 0.15% oxygen.
The element and its compounds make up 49.2%, by weight, of the earth’s crust.
About two thirds (2/3rd) of the human body and nine tenths (9/10th) of water is oxygen.
Apart from the inert gasses (helium, neon, argon and krypton), Approx every chemical is bind with oxygen to form compounds.
Main component of the sand (Water (H2O) and Silica (SiO2)) are among the more abundant binary oxygen compounds.
Among the compounds which contain more than two elements, silicates are the most abundant, that form most of the rocks and soils.
Other compounds which are abundant in nature are Calcium carbonate (limestone and marble, CaCO3), Aluminum oxide (bauxite, Al2O3.nH2O), Calcium sulphate (gypsum, CaSO4.2H2O), and various iron oxides, that are used as source of the metal.
Natural occurring oxygen-18 is stable and available commercially, as is water (H2O with 15% 18O).
Methods used to obtain oxygen gas
The first is by the Distillation of liquid air.
The second is to pass clean, dry air through a Zeolite (hydrated aluminum silicate, NaAlSi2O6-H2O) that absorbs nitrogen and leaves oxygen.
A newer method, which gives oxygen of a higher purity, is to pass air over a partially permeable ceramic membrane.
In the laboratory it can be prepared by the electrolysis of water or by heating potassium chlorate (KClO3) with manganese dioxide (MnO2) as a catalyst to aqueous hydrogen peroxide (H2O2).
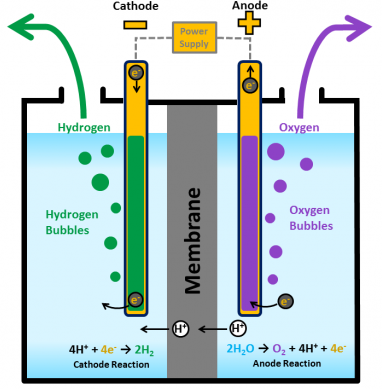
Air separation plants produce about 99% of the oxygen gas, while electrolysis plants produce about 1%.
Annual world wide production is around 100,000,000 (100 million) tonnes.
1 % (In Universe)
40% (In Meteorites)
0.9% (In Sun)
46% (In Earth’s Crust)
86% (In Oceans)
61% (In Humans)
Oxygen Price
Pure (99%) element price is around $10-$20 per Litre.
Oxygen Element Database
Atomic Spectroscopic Data
→ ASD Line
→ ASD Levels
→ Ground States and Ionization Energies
→ Handbook of Basic ASD
Atomic and Molecular Data
→ Electron-Impact Cross Sections
Bibliographic Databases on Atomic Spectroscopy
→ Atomic Transition Probability Bibliographic Database
→ Atomic Spectral Line Broadening Bibliographic Database
→ Atomic Energy Levels and Spectra Bibliographic Database
X-Ray and Gamma Ray Data
→ X-ray Attenuation and Absorption for Materials of Dosimetric Interest
→ XCOM: Photon Cross Section Database
→ Form Factor, Attenuation, and Scattering Tabulation
Radiation Dosimetry Data
→ Electrons
→ Helium Ions
→ Protons
Nuclear Physics Data
Condensed Matter Physics Data
→ Atomic Reference Data for Electronic Structure Calculations
References
Wikipedia
Los Alamos National Laboratory
National Institute of Standards and Technology
Environmental Chemistry
Royal Society of Chemistry
Periodic Table
Lenntech
Web Elements
Michael Pilgaard’s Elements