07 N (Nitrogen Element)

CONTENT INDEX
About Nitrogen
Nitrogen, as a gas is Colorless, Odorless, Tasteless and generally considered an inert element.
As a liquid (At BP = -195.8°C), it is also Colorless and Odorless, and appearance is similar as water.


Identity
CAS Number: CAS7727-37-9
CID Number: CID947
DOT Hazard Class: 2.1
DOT Number: 1977
RTECS Number: RTECSQW9700000
Properties of Nitrogen Element
Basic Properties of Nitrogen
Pronunciation: nai.tra.zan
Allotrops: Dinitrogen (N2)
Appearance: Colorless gas, liquid or solid
Mass Number: 14
Standard Atomic weight: 14.007 g/mol
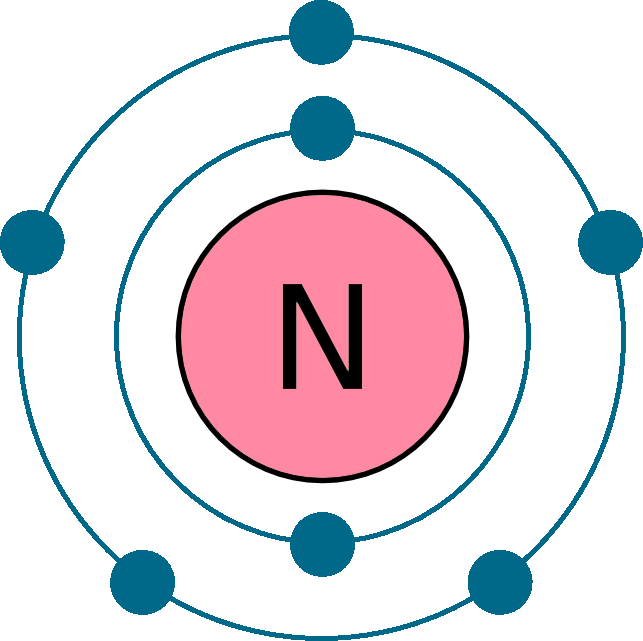
Atomic number (Z): 7
Electrons: 7
Protons: 7
Neutrons: 7
Period: 2
Group: 15
Block: p
Element category: Reactive Nonmetal
Electrons per shell: K2, L5
Electron configuration: 1s22s22p3
Thermal Properties of Nitrogen
Phase: Gas
Melting point: 63.15 K (-210 oC, -346 oF)
Boiling point: 77.35 K (-195.79 oC, -320.43 oF)
Debye temperature: N/A
Triple point Temperature: 63.15 K (-210 oC, -346 oF)
Triple point Pressure: 12.52 kPa (0.1235 Atm)
Critical point Temperature: 126.21 K (-146.94 oC, -232.492 oF)
Critical point Pressure: 3.39 MPa (33.457 Atm)
Fusion heat: 0.72 kJ/mol
Vaporization heat: 5.56 kJ/mol
Specific heat: 1040 J/(kg K)
Molar heat capacity: 29.124 J/(mol.K)
Thermal expansion: N/A
Thermal conductivity: 25.83×10-3 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: N/A
Electrical Properties of Nitrogen
Electrical conductivity: N/A
Electrical resistivity: N/A
Electrical type: N/A
Critical point (Superconducting point): N/A
Magnetic Properties of Nitrogen
Magnetic type: Dimagnetic
Curie point: N/A
Magnetic susceptibility (xmol): –N/A
Volume magnetic susceptibility: -6.8×10-9
Mass magnetic susceptibility: -5.4×10-9 m3/kg
Molar magnetic susceptibility: -0.15×10-10 m3/mol
Physical Properties of Nitrogen
Density: 1.250 g/L (At STP) 0.808 g/cm3 (In Liquid at B.P)
Molar volume: 0.011197 m3/mol
Young’s modulus: N/A
Shear modulus: N/A
Mohs Hardness: N/A
Bulk modulus: N/A
Poisson ratio: N/A
Vicker hardness: N/A
Brinell hardness: N/A
Refractive Index: 1.000298
Sound Speed: 353 m/s (In gas)
Atomic Properties of Nitrogen
Oxidation states: +3,+4,+3, +2, +1, -1, -2, -3
Valence Electrons: 2s2 2p3
Ion charge: N3+
The ionization potential of an atom: 14.48 eV
Ionization energies: 1st: 1402.2 kJ.mol 2nd: 2856 kJ/mol 3rd: 4578.12 kJ/mol
Ionic radius: 13 pm
Atomic radius: 92 pm (empirical)
Van der Waals: 155 Pm
Covalent radius: 71±1 pm
Filling Orbital: 2p3
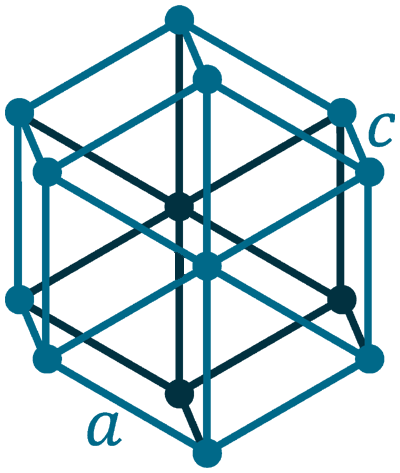
Crystal structure: Hexagonal
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 386.1, 386.1, 626.5 pm
Grid parameters: a(H)=3.86 Å c(H)=6.26 Å
Attitude c/a: N/A
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Nitrogen
Electronegativity: 3.04 (pauling scale)
Valence: +3
Electron affinity: 7 kJ/mol
Nuclear Properties of Nitrogen
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 4S3/2
Neutron cross section (Brans): 1.91
Neutron Mass Absorption: 0.0048
Isotopes: 13N 14N 15N
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 13N | Syn | – | 9.964 min |
| 14N | 99.6% | 14.002 | Stable |
| 15N | 0.4% | 15.000 | Stable |
Chemical Reactions of Nitrogen Element
Nitrogen dosen’t reacts with Air, Water, Halogens, Acids, and Bases.
Nitrogen Reaction With Hydrogen
N2 can be brought to react with hydrogen, is called the Haber process
N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g)
Nitrogen History
Naming: Name comes from the Greek words Nitron genes meaning Nitre and forming and the Latin word Nitrum
Discovery: Daniel Rtherford (1772)
Named By: Jean Antoine Chaptal (1790)
Nitrogen Uses
Nitrogen is very important to the chemical industry, and a greatly commercial use of it as a component in the manufacture of Ammonia (NH3) by the Haber process and also used to make Fertilisers, Poisons [Cyanides (CN-)], Potassium nitrate (KNO3), Ammonium nitrate (NH4NO3), Nitric acid (HNO3), Nylon [(C12H22N2O2)n], Dyes and Explosives (such as NitroGlycerine (C3H5N3O9) and Trinitrotoluene [TNT, (C7H5N3O6)].
Nitrogen gas (Inert gas) is also used to provide an nonreactive atmosphere, in this way it is used to preserve foods, and in the electronics industry during the production of Transistors and Diodes.
A great quantity of nitrogen is used in Annealing process (heat treatment) of stainless steel and other steel mill products.
Liquid nitrogen (LN2) is often used as as a Refrigerant for freezing and Transporting food products to maintain moisture, colour, flavour and texture, and also used for preservation of Bodies and Reproductive cells (sperm and eggs) for medical research and reproductive technology.
Biological Role Of Nitrogen Element
Liquid nitrogen temperature is very low, so it should be handled with care.
Nitrogen is cycled naturally by living organisms through the ‘nitrogen cycle’, which is one of the most important processes in nature for living organisms.
Bacteria in the soil are capable of “fixing” the nitrogen into a usable form (as a fertilizer) for plants by convert the waste nitrogen compounds back to nitrogen gas.
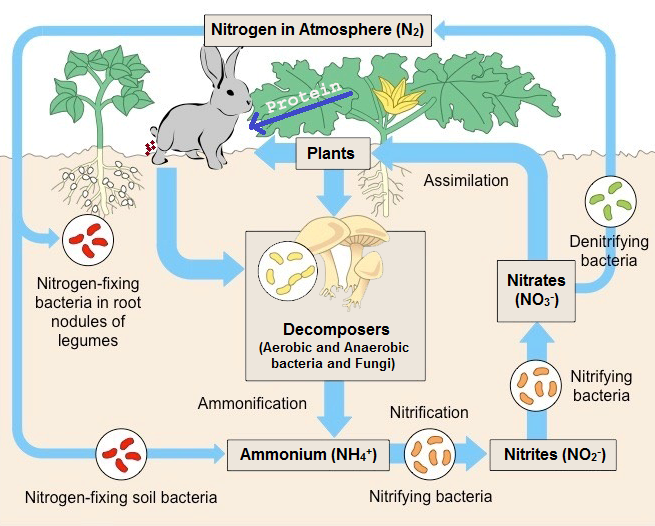
Nitrogen is an essential element for life, because it is a constituent of DNA, which is a part of the genetic code.
Nitrogen is taken up by green plants and algae as nitrates (NO3–), and used to build up the bases needed to construct DNA (Deoxyribonucleic acid ), RNA and all Amino acids (building blocks of proteins).
Animals obtain their nitrogen by consuming other living things (animals, plants etc..).
Microbes (microorganism) in the soil convert the nitrogen compounds back to nitrates (NO3–) for the plants to re-use.
The nitrate supply is also replenished (fill up) by nitrogen-fixing bacteria that ‘fix’ nitrogen directly from the atmosphere.
The crop yields can be more increased by adding chemical fertilizers to the soil, manufactured from Ammonia (NH3), but If it is used carelessly, then the fertilizer can leach out of the soil into Lakes and Rivers, causing algae to grow rapidly. And This can block out light preventing photosynthesis, due to this dissolved oxygen soon gets used up and the Lake or River dies.
Health Effects
Nitrates (NO3–) and Nitrites (NO2-‑) causes several Health effects, as follows:
→ Reactions with hemoglobin (iron-containing oxygen) in blood, causing the oxygen carrying capacity of the blood to decrease (Nitrite)
→ Shortages of Vitamin A (Nitrate)
→ Decreased functioning of the thyroid gland (Nitrate)
→ Fashioning of Nitro amines, which are known as one of the most common causes of Cancer (Nitrates and Nitrites)
Nitric oxide (NO) is much more important than nitrogen alone for metabolic.
It was a vital body messenger for relaxing muscles, and today it is involved in the immune system, the cardiovascular system, the central nervous system, and the peripheral nervous system.
The enzyme that produces Nitric oxide (NO) is called Nitric oxide synthesis, and it is abundant in the brain. Although Nitric oxide (NO) is relatively short-lived, it can diffuse through membranes to carry out its functions.
Nitric oxide activates an erection by relaxing the muscle, that controls the blood flow into the penis, and The medicine / drug Viagra works by releasing Nitric oxide to produce the same effect.
Abundance of Nitrogen Element
By volume, Nitrogen (N2) makes up 78.1% of the Earth’s Atmosphere and only 2.6% nitrogen of the Mars atmosphere.
Nitrogen is found in all living systems as part of the makeup of biological compounds and also in coal and other fossil fuels.
Nitrogen molecules mainly occur in air. In water and soils nitrogen can be found in Nitrites (NO2–) and Nitrates (NO3–), All of these substances are a part of the Nitrogen cycle, and there are all interconnected.
From an exhaustible source in our atmosphere, nitrogen gas can be obtained by Liquefaction (generates a liquid from a solid or a gas) and fractional Distillation (it is a technique used to separate chemical mixture into different parts according to their boiling points to produce high purity distillates) of liquid air.
Nitrogen gas can be prepared by heating a water solution of Ammonium Nitrite (NH4NO3).
Annual world wide extraction is around 45,000,000 tonnes
0.1% (In Universe)
0.14% (In Meteorites)
0.1% (In Sun)
0.002% (In Earth’s Crust)
0.00005% (In Oceans)
2.6% (In Humans)
Nitrogen Element Price
Pure (99%) Liquid Nitrogen price is around $0.5-$1.5 per Litre, But Cryogenic Container is very costly.
You Can Buy Pure Nitrogen Gas and Product
Element Database
Atomic Spectroscopic Data of Nitrogen
→ ASD Line
→ ASD Levels
→ Ground States and Ionization Energies
→ Handbook of Basic ASD
Atomic and Molecular Data of Nitrogen
→ Electron-Impact Cross Sections
Bibliographic Databases on Atomic Spectroscopy of Nitrogen
→ Atomic Transition Probability Bibliographic Database
→ Atomic Spectral Line Broadening Bibliographic Database
→ Atomic Energy Levels and Spectra Bibliographic Database
X-Ray and Gamma Ray Data of Nitrogen
→ X-ray Attenuation and Absorption for Materials of Dosimetric Interest
→ XCOM: Photon Cross Section Database
→ Form Factor, Attenuation, and Scattering Tabulation
Radiation Dosimetry Data of Nitrogen
→ Electrons
→ Helium Ions
→ Protons
Nuclear Physics Data of Nitrogen
Condensed Matter Physics Data of Nitrogen
→ Atomic Reference Data for Electronic Structure Calculations
References
Wikipedia
Los Alamos National Laboratory
National Institute of Standards and Technology
Environmental Chemistry
Royal Society of Chemistry
Periodic Table
Lenntech
Web Elements
Michael Pilgaard’s Elements




This post is more than enough for those who want to know about Nitrogen element. Thanks for sharing an informative post.
share this information with others…thank you..