25 Mn (Manganese Element)

It is gray-white, chemically active element, resembling (look or seem like) iron, but it is harder and very brittle.
Pure manganese is chemically reactive, It is hard to melt, but easily oxidized and decomposes slowly in cold water.
As a powder, it will burn in oxygen and dissolves in dilute acids.
Pure metal exists in four allotropic forms, where the α-alpha form is stable at ordinary temperature; γ-gamma manganese changes to α-alpha at ordinary temperatures is said to be soft, flexible, easily cut, and capable of being bent.
AManganese improves forging and rolling qualities in steel, along with adding strength, hardness, stiffness, wear resistance.

Identity
CAS Number: CAS7439-96-5
CID Number: CID23930
DOT Hazard Class: 4.1
DOT Number: 3089
CONTENT INDEX
Basic Properties of Manganese
Pronunciation: Mang-ga-neez
Appearance: Silvery Metallic
Mass Number: 55
Standard Atomic weight: 54.938 g/mol
Atomic number (Z): 25
Electrons: 25
Protons: 25
Neutrons: 30
Period: 4
Group: 7
Block: d
Element category: Transition metal
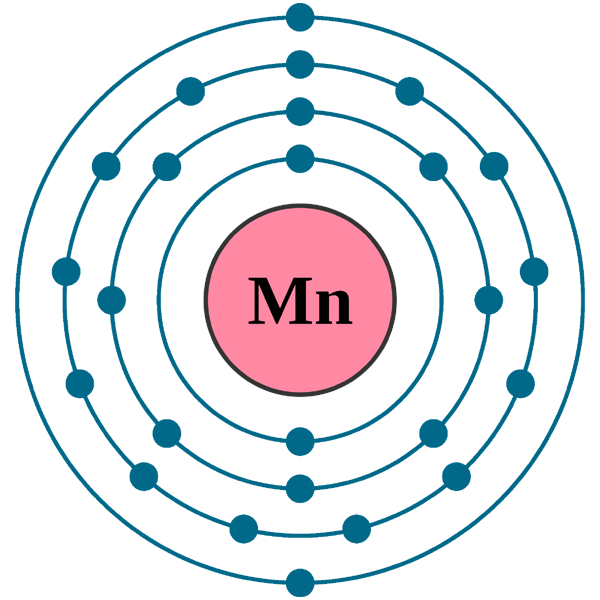
Electrons per shell: K2, L8, M13, N2
Electron configuration: 1s22s22p63s23p63d54s2
Thermal Properties of Manganese
Phase: Solid
Melting point: 1519 K (1246 oC, 2275 oF)
Boiling point: 2334 K (2061 oC, 3742 oF)
Debye temperature: 400 K (126.85 oC, 260.33 oF)
Fusion heat: 12.91 kJ/mol
Vaporization heat: 221 kJ/mol
Specific heat: 479 J/(kg K)
Molar heat capacity: 26.32 J/(mol.K)
Thermal expansion: 21.7 μm/(m∙K)
Thermal conductivity: 7.81 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: 100 K (173.15 oC, -279.67 oF) (Temperature, above which an antiferromagnetic material becomes paramagnetic)
Electrical properties of Manganese
Electrical conductivity: 620000 S/m
A Electrical resistivity: 1.44 μΩ∙m
A Electrical type: Conductor
Magnetic Properties of Manganese
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +529×10-6 cm3/mol (α)
Volume magnetic susceptibility: 0.00090387
Mass magnetic susceptibility: 121×10-9 m3/kg
Molar magnetic susceptibility: 6.6475×10-9 m3/mol
Physical Properties of Manganese
Density: 7.21 g/cm3 (In solid) 5.95 g/cm3 (In Liquid at M.P)
Molar volume: 0.0000073545 m3/mol
Young’s modulus: 198 GPa
Mohs Hardness: 6.0
Bulk modulus: 120 GPa
Brinell hardness: 196 MPa
Sound Speed: 5150 m/s
Atomic Properties of Manganese
Oxidation states: -3, -2, -1, 1, 2, 3, 4, 5, 6, 7
Valence Electrons: 3d5 4s2
Ion charge: Mn2+ Mn4+
Ionization potential of an atom: 7.40
Ionization energies: 1st: 717.3 kJ.mol 2nd: 1509 kJ/mol 3rd: 3248 kJ/mol
Ionic radius: 46 pm
Atomic radius: 127 pm (empirical)
Van der Waals: 197 Pm
Covalent radius: 139±5 pm (Low spin), 161±8 pm (High spin)
Filling Orbital: 3d5
Crystal structure: Body centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 889.0, 889.0, 889.0 pm
Grid parameters: a=8.890 Å
Space Group Name: I_43m
Space Group Number: 217

Reactivity of Manganese
Electronegativity: 1.55 (pauling scale)
Valence: +4
Electron affinity: 0 kJ/mol
Nuclear Properties of Manganese
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 6S5/2
Neutron cross section (Brans): 13.3
Neutron Mass Absorption: 0.0083
Isotopes: 52Mn 53Mn 54Mn 55Mn
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 52Mn | Syn | – | 5.6 d |
| 53Mn | Trace | – | 3.74×106 y |
| 54Mn | Syn | – | 312.03 |
| 55Mn | 100 | 54.938 | Stable |
Chemical Reactions of Manganese
Manganese is not very reactive in air, but the surface of manganese lumps oxidizes a little, and when finely divided manganese metal burns in air, It forms:
3 Mn (s) + 2 O2 (g) → Mn3O4 (s) (manganese oxide)
3 Mn (s) + N2 (g) → Mn3N2 (s) (manganese nitride)
It Reacts slowly with water:
Mn(s) + 2 H2O (l) ⇌ MnO2 (s) + 4 H+ (aq) + 4 e– E° = -0.024 V
Mn2 +(aq) + 4 H2O (l) ⇌ MnO42- (aq) + 8 H+ (aq) + 4e– E° = -1.742 V
Mn2 +(aq) + 4 H2O (l) ⇌ MnO4– (aq) + 8 H+ (aq) + 5 e– E° = -1.507 V
3 MnO42- (aq) [green] + 2 H2O (l) ⇌ 2 MnO4– (aq) [purple] + 4 OH– (aq) + MnO2 (s)
Reacts with the halogens, and forming manganese(II) halides, But for fluoride, manganese(III)fluoride is also formed.
Mn (s) + F2 (g) → MnF2 (s) (Manganese (II) fluoride)
2 Mn (s) + 3 F2 (g) → 2 MnF3 (s) (Manganese (III) fluoride)
Mn (s) + Cl2 (g) → MnCl2 (s) (Manganese (II) chloride)
Mn (s) + Br2 (g) → MnBr2 (s) (Manganese (II) Bromide)
Mn (s) + I2 (g) → MnI2 (s) (Manganese (II) Iodide)
Manganese (II) ions are readily oxidized to MnO2 by bromine under alkaline conditions:
Mn2+ (aq) + Br2 (aq) + 2 OH– (aq) → MnO2 (s) [brown-black] + 2 HBr (aq)
AManganese with oxidation steps >2 will be reduced to Mn (II) by Br– and I– under acidic conditions under the formation of Br2 and I2 respectively, Let’s take an e.g:
MnO2 (s) + 2 Br– (aq) + 4 H+ (aq) → Mn2+ (aq) + Br2 (aq) + 2 H2O (I)
MnO2 (s)m+ 2 I– (aq) + 4 H+ (aq) → Mn2+ (aq) + I2 (aq) + 2 H2O (I)
The metal dissolves readily in dilute sulphuric acid, and forming a colorless solution of Mn(II) ions and hydrogen gas, where in this aqueous solution the Mn(II) is present as the virtually colourless complex ion [Mn(OH2)6]2+.
Mn (s) + H2SO4 (aq) → Mn2+ (aq) + SO42- (aq) + H2 ↑ (g)
Manganese History
Naming: Latin: mangnes (magnet); Ital. manganese.
Discovery: Carl Wilhelm Scheele (1774)
First isolation: Johann Gottlieb Gahn (1774)
Manganese Uses
AManganese is essential to iron and steel production, and It is mainly used in alloys (such as steel), which contains 1% Mn to increase the strength and also improve workability and resistance to wear.
Manganese steel contains around 13% manganese, which is extremely strong and is used for railway tracks, safes, rifle barrels and prison bars.
Mostly Drink cans are made of aluminium alloy with 1.6% manganese, to improve corrosion resistance.
AMaganese can also be alloyed with other metals such as Aluminum(Al), Antimony (Sb) or Copper (Cu) to form highly ferromagnetic (form permanent magnets) alloys.
Manganese(IV) oxide (MnO2) is used as a catalyst , a rubber additive, as a depolarizer in dry cells, and to decolourise glass that is coloured green by impurities of Iron.
It is also used in the preparation of chlorine and oxygen and in drying black paints.
Manganese sulfate (MnSO4) is used to make a fungicide (a chemical that destroys fungus).
Manganese (II) oxide (MnO) is a powerful oxidising agent, so it is used in quantitative analysis, and in medicine, and also used to make fertilisers and ceramics.
Biological role of Manganese
AManganese is an essential element in all known living organisms for development, metabolism, and the antioxidant system.
There are many types of enzymes contain manganese, like the enzyme responsible for converting water molecules to oxygen during photosynthesis contains four atoms of amanganese.
Some soils have low levels of manganese and so it is added to some fertilisers and given as a food supplement to grazing (grassland suitable for pasturage) animals.
The average human body contains about 12 mg (milligrams) of manganese, and we intake about 4 mg each day from such foods as nuts, wholegrain cereals, bran, tea and parsley.
Because without it bones grow spongier (compressible) and break more easily, and It is also essential for the utilization of vitamin B1.
But manganese fumes, dusts, and compounds should not exceed the ceiling value (5 mg/m3), even for short periods, because of the element’s toxicity level.
Abundance of Manganese
It is the 5th most abundant metal in the Earth’s crust.
Manganese minerals are widely distributed with Pyrolusite (manganese dioxide, MnO2), Tephroite (manganese silicates, MnO3Si), and rhodochrosite (manganese carbonates, MnCO3) being the most common.
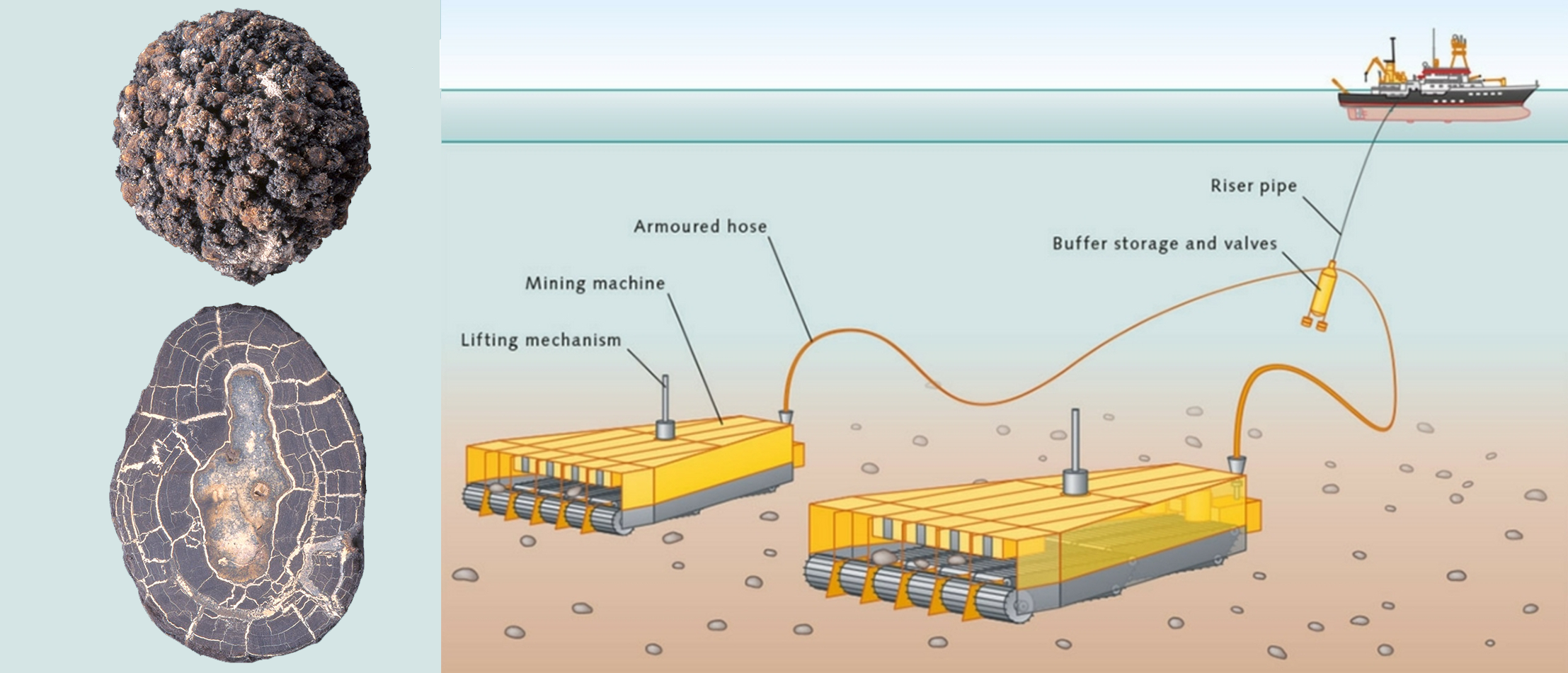
Large quantities of manganese nodules (around 500 billion tons) are found on the ocean floor, and it contain about 24% amanganese, together with many other elements in lesser abundance.
Commercialy, the metal is obtained by reducing the oxide with sodium, magnesium or aluminium, or by the electrolysis.
Annual world wide production is around 8,000,000 tons.
5×10-7% (In Universe)
1.7×10-5% (In Meteorites)
1×10-7% (In Sun)
0.0008% (In Earth’s Crust)
4.5×10-11% (In Oceans)



World’s Top 3 producers of Manganese
1) China
2) South Africa (Gabon)
3) Australia
World’s Top 3 Reserve holders of Manganese
1) South Africa
2) Ukraine
3) Brazil
#Manganese


