57 La (Lanthanum)

Lanthanum is a soft,ductile, malleable, silver-white metal, which can be with a knife.
It is very reactive, and it oxidizes rapidly in the air.
It decomposes slowly in cold water and rapidly in hot water.
The metal reacts directly with elemental carbon, boron, silicon, nitrogen, sulfur, selenium, phosphorus, and with halogens. It is easily ignited.

Identity
CAS Number: CAS7439-91-0
CID Number: CID23926
DOT Hazard Class: 4.1
DOT Number: 3178
CONTENT INDEX
Basic Properties of Lanthanum
Pronunciation: Lan-tha-nam
Appearance: Silvery white
Mass Number: 139
Standard Atomic weight: 138.905 g/mol
Atomic number (Z): 57
Electrons: 57
Protons: 57
Neutrons: 82
Period: 6
Block: d
Element category: Lanthanide
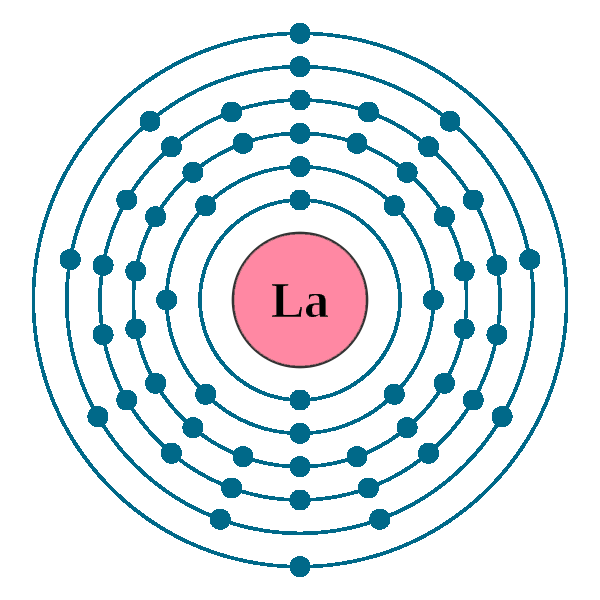
Electrons per shell: K2, L8, M18, N18, O9, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p65d16s2
Thermal Properties of Lanthanum
Phase: Solid
Melting point: 1193 K (920 oC, 1688 oF)
Boiling point: 3737 K (3464 oC, 6267 oF)
Debye temperature: 132 K (-141.15 oC, -222.07)
Fusion heat: 6.20 kJ/mol
Vaporization heat: 400 kJ/mol
Molar heat capacity: 27.11 J/(mol.K)
Thermal expansion: α, poly: 12.1 μm/(m∙K)
Thermal conductivity: 13.4 W/(m∙K)
Neel Point (magnetic ordering temperature) TN: 106 K (Temperature, above which an antiferromagnetic material becomes paramagnetic)
Electrical properties of Lanthanum
Electrical conductivity: 1.6×106 S/m
A Electrical resistivity: α, poly: 615 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 4.88 K (-268.27 oC, -450.89 oF)
Magnetic Properties of Lanthanum
A Magnetic type: Paramagnetic
Magnetic susceptibility (xmol): +118×10-6 cm3/mol
Volume magnetic susceptibility: 0.00006761
Mass magnetic susceptibility: 11×10-9 m3/kg
Molar magnetic susceptibility: 1.528×10-9 m3/mol
Physical Properties of Lanthanum
Density: 6.162 g/cm3 (In solid) 5.94 g/cm3 (In Liquid, at m.p)
Molar volume: 0.0000226 m3/mol
Young’s modulus: α form: 36.6 GPa
Shear modulus: α form: 14.3 GPa
Mohs Hardness: 2.5
Bulk modulus: α form: 27.9 GPa
Poisson ratio: α form: 0.280
Vicker hardness: 360-1750 MPa
Brinell hardness: 350-400 MPa
Sound Speed: 2475 m/s
Atomic Properties of Lanthanum
Oxidation states: 3,2,1
Valence Electrons: 5d1 6s2
Ion charge: La3+
Ionization energies: 1st: 538.1 kJ.mol 2nd: 1067 kJ/mol 3rd: 1850.3 kJ/mol
Ionic radius: 106.1 pm
Atomic radius: 240 pm (Van der Waals)
Covalent radius: 207±8 pm
Filling Orbital: 5d1
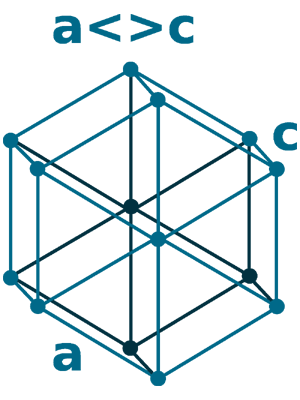
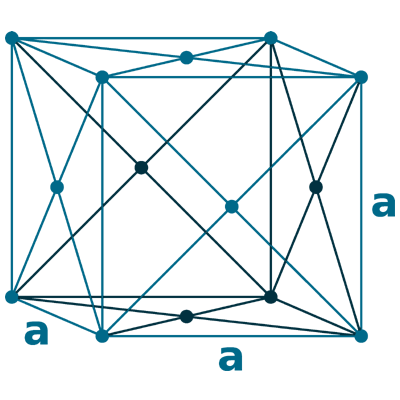
Crystal structure: Double hexagonal close packed (At Room temperature), Changed to Face-centered cubic (At 310 oC), Body-centered cubic (At 865 oC)
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 377.2, 377.2, 1214.4 pm
Grid parameters: a=3.772 Å c=12.14 Å
Attitude c/a: 3.22
Space Group Name: P63/mmc
Space Group Number: 194

Reactivity of Lanthanum
Electronegativity: pauling scale: 1.10
Valence: +3
Electron affinity: 48 kJ/mol
Nuclear Properties of Lanthanum
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2D3/2
Neutron cross section (Brans): 8.98
Neutron Mass Absorption: 0.0023
Isotopes: 137La 138La 139La
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 137La | Syn | – | 6×104 y |
| 138La | 0.089 | 137.907 | 1.05×1011 y |
| 139La | 99.911 | 138.906 | Stable |
Lanthanum History
Naming: From the Greek ‘lanthanein’ (meaning, to lie hidden).
Discovery: Carl Gustaf Mosander (1838)
Lanthanum Uses
Mischmetal Is an alloy (Containing 50% cerium, 25% lanthanum, Small amounts of Neodymium and Praseodymium), which is used in making cigarette lighter flints.
It is extensively used in carbon arc lighting applications, Such as in the motion picture industry for studio lighting and projection.
Lanthanum oxide (La2O3) improves the alkali resistance of glass, and used to make special optical glasses (Infrared absorbing glass, camera lenses, telescope lenses).
Small amounts of lanthanum, as an additive, can be used to improves the malleability and resistance of steel and to produce nodular cast iron.
Lanthanum alloyed with nickel is used to store hydrogen gas for use in hydrogen-powered vehicles. It is also found in the anode of nickel metal hydride batteries, which is used in hybrid cars.
Hydrogen sponge alloys containing lanthanum, have possibilities in an energy conservation system. Because The process is reversible, where this alloys take up to 400 times their own volume of hydrogen gas and every time heat energy is released, when they take up the gas.
Lanthanum salts are used in zeolite catalysts for petroleum refining, because it stabilize the zeolite at high temperature.
Lanthanum ion La3+ is used as a biological tracer for Calcium ion Ca2+, and radioactive lanthanum has been tested for use in treating cancer.
Biological role: It has Low to moderate toxicity, it should be handled with care.
Abundance of Lanthanum
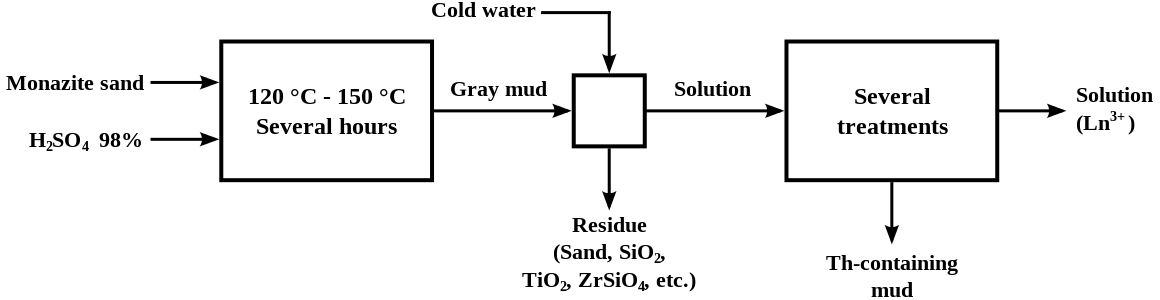
Lanthanum is Found in various minerals like Allanite (orthite), monazite, bastnasite, cerite. The Most important sources are Monazite (25% Lanthanum) and bastnasite (38% Lanthanum).
It commercially extracted by ion exchange and solvent extraction techniques.
The metal can be usually obtained by reducing the anhydrous fluoride with calcium.
Annual world wide production is around 12,500 tons.
2×10-7% (In Universe)
2.8×10-5% (In Meteorites)
2×10-7% (In Sun)
0.0034% (In Earth’s Crust)
3.4×10-10% (In Oceans)
World’s Top 3 producers of Lanthanum
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders of Lanthanum
1) China
2) CIS Countries (inc. Russia)
3) USA
#lanthanum


