67 Ho (Holmium)

Holmium has a metallic to bright silver luster, soft and Malleable.
It is rapidly oxidizes in moist air and at elevated temperatures, But stable in dry air at room temperature (20 oC)

Identity
CAS Number: CAS7440-60-0
CID Number: CID23988
DOT Numbers: 3089
CONTENT INDEX
Basic Properties of Holmium
Pronunciation: Hol-mee-am
Appearance: Silvery white
Mass Number: 164
Standard Atomic weight: 164.930 g/mol
Atomic number (Z): 67
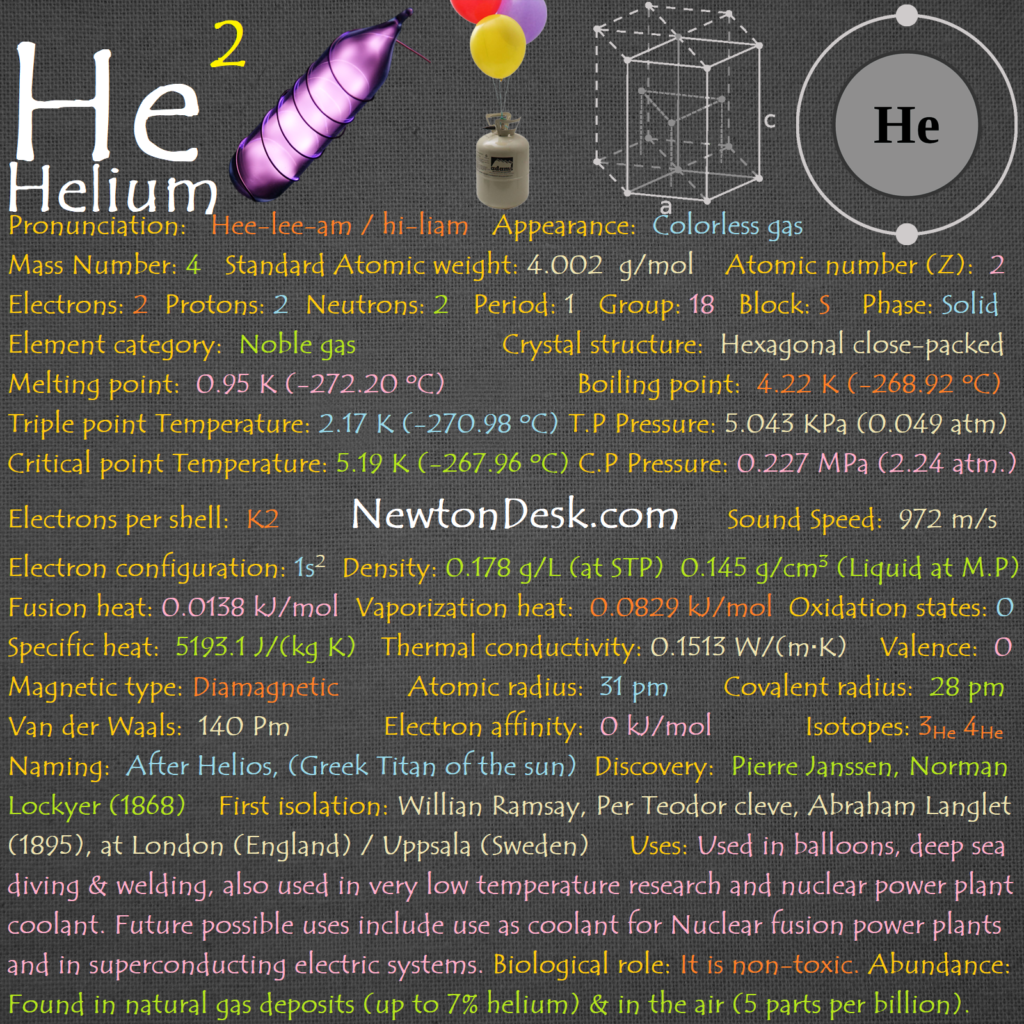
Electrons: 67
Protons: 67
Neutrons: 97
Period: 6
Block: f
Element category: Lanthanide
Electrons per shell: K2, L8, M18, N29, O8, P2
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f116s2
Thermal Properties of Holmium
Phase: Solid
Melting point: 1734 K (1461 oC, 2662 oF)
Boiling point: 2873 K (2600 oC, 4712 oF)
Fusion heat: 17 kJ/mol
Vaporization heat: 251 kJ/mol
Molar heat capacity: 27.15 J/(mol.K)
Thermal expansion: poly: 11.2 μm/(m∙K)
Thermal conductivity: 16.2 W/(m∙K)
Neel Point: 132 K
Electrical properties of Holmium
Electrical conductivity: 1.1 x106 S/m
s Electrical resistivity: poly: 0.814 μΩ∙m
s Electrical type: Conductor
Curie point: 20 K
Magnetic Properties of Holmium
Magnetic type: Paramagnetic
s Volume magnetic susceptibility: 0.0482845
Mass magnetic susceptibility: 5490×10-9 m3/kg
Molar magnetic susceptibility: 905.467×10-9 m3/mol
Physical Properties of Holmium
Density: 8.79 g/cm3 (In solid) 8.34 g/cm3 (In Liquid)
Molar volume: 0.00001875 m3/mol
Young’s modulus: 64.8 GPa
Shear modulus: 26.3 GPa
Mohs Hardness: 1.65
Bulk modulus: 40.2 GPa
Poisson ratio: 0.231
Vicker hardness: 410-600 MPa
Brinell hardness: 500-1250 MPa
Sound Speed: 2760 m/s
Atomic Properties of Holmium
Oxidation states: 3, 2, 1
Valence Electrons: 4f11 6s2
Ion charge: Ho3+
Ionization energies: 1st: 581.0 kJ.mol 2nd: 1140 kJ/mol 3rd: 2204 kJ/mol
Ionic radius: 90.1 pm
Atomic radius: 216 pm (Van der Waals)
Covalent radius: 192±7 pm
Filling Orbital: 4f11
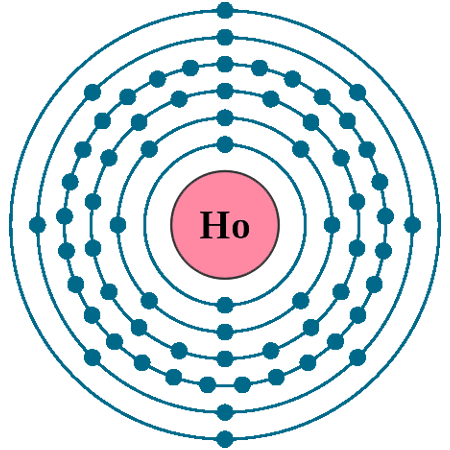
Crystal structure: Hexagonal close-packed
Lattice angles: π/2, π/2, π/3
Lattice constant: 357.73, 357.73, 561.6 pm
Grid parameters: a=3.577 Å, c=5.616 Å
Attitude c/a: 1.570
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Holmium
Electronegativity: pauling scale: 1.23
Valence: +3
Electron affinity: 50 kJ/mol
Nuclear Properties of Holmium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 4I15/2
Neutron cross section (Brans): 65
Neutron Mass Absorption: 0.015
Isotopes: 163Ho 164Ho 165Ho 166Ho 167Ho
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 163Ho | Syn | – | 4570 y |
| 164Ho | Syn | – | 29 min |
| 165Ho | 100 | 164.93 | Stable |
| 166Ho | Syn | – | 26.763 h |
| 167Ho | Syn | – | 3.1 h |
Chemical Reactions
Holmium metal tarnishes slowly in air and burns readily to form a holmium (lll) oxide:
4 Ho + 3 O2 → 2 Ho2O3
Reacts slowly with cold water and rapidly with hot water (form holmium hydroxide and hydrogen gas):
2 Ho (s) + 6 H2O (l) → 2 Ho(OH)3 (aq) + 3 H2 (g)
The metal reacts with all Halogens to form Dysprosium (lll) halides:
2 Ho (s) + 3 F2 (g) → 2 HoF3 (s) [pink] (Holmium (lll) fluoride)
2 Ho (s) + 3 Cl2 (g) → 2 HoCl3 (s) [yellow] (Holmium (lll) chloride)
2 Ho (s) + 3 Br2 (g) → 2 HoBr3 (s) [yellow] (Holmium (lll) bromide)
2 Ho (s) + 3 I2 (g) → 2 HoI3 (s) [yellow] (Holmium (lll) iodide)
Dissolves readily in dilute sulfuric acid to form Solutions containing Holmium (lll) ions (yellow):
2 Ho (s) + 3 H2SO4 (aq) → 2 Ho3+ (aq) + 3 SO42− (aq) + 3 H2 (g)
Holmium History
Naming: From Holmia (Greek name of Sweden)
Discovery: Jacques-Louis Soret (1878)
Holmium Uses
AHolmium can readily absorb neutrons, So it is used As control-rods in nuclear reactors to keep a chain reaction under control.
Its alloys are used as a flux concentrator for high magnetic fields.
Biological role: It is Low-toxic, But it should be handled with care.
Abundance of Holmium
Holman’s Chief ores are monazite and bastnasite.
It is extracted from those ores that are processed to extract yttrium.
It is obtained by ion exchange and solvent extraction.
Annual world production is around 10 tons.
5×10-8% (In Universe)
5.9×10-6% (In Meteorites)
0.00012% (In Earth’s Crust)
2.2×10-11% (In Oceans)
World’s Top 3 producers of Holmium
1) China
2) Russia
3) Malaysia
World’s Top 3 Reserve holders of Holmium
1) China
2) CIS Countries (inc. Russia)
3) USA
#holmiun