79 Au (Gold)

Of all the elements, Apparence of pure gold (24 carat) is the most beautiful.
It is soft, metallic, having yellow colour, and when finely divided, it may be convert to black, ruby, or purple colour, where the Purple of Cassius is a delicate test for auric gold (Au3+).
It is the most ductile & malleable metal, even 28 gm (1 ounce) of gold can be beaten upto 300 square feet.
The metal is a good conductor of heat & electricity, and Also inert (unaffected by air and most reagents).
Due to its softness, It is usually alloyed to give it more strength.
The most common gold compounds are Chlorauric acid (HAuCl4) & Auric chloride (AuCl3).
It is Unreactive with air & most reagents, But It is dissolved in aqua regia (mixture of nitric & hydrochloric acid in ratio of 1:3).

Identity
CAS Number: CAS7440-57-5
CID Number: CID23985
RTECS Number: RTECSMD5070000
CONTENT INDEX
Basic Properties of Gold
Appearance: Metallic yellow
Mass Number: 197
Standard Atomic weight: 196.966 g/mol
Atomic number (Z): 79
Electrons: 79
Protons: 79
Neutrons: 118
Period: 6
Group: 11
Block: d
Element category: Transition metal
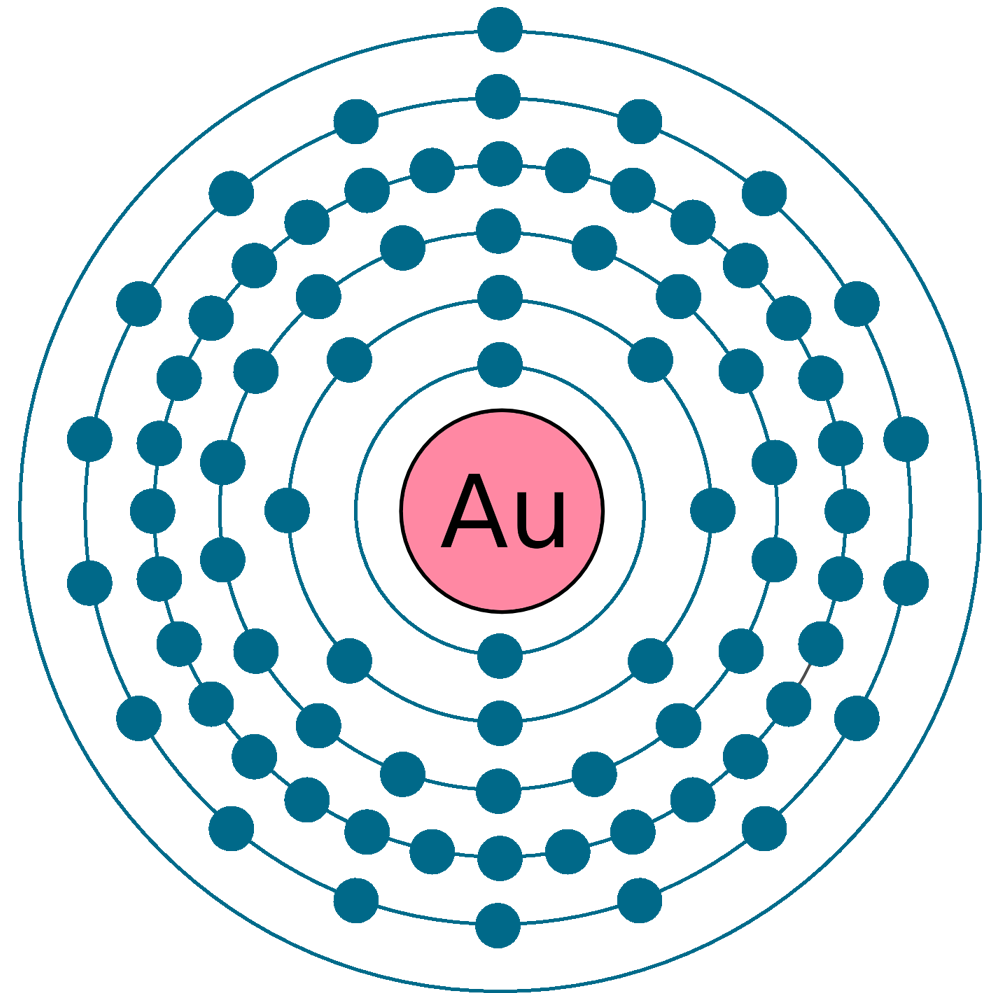
Electrons per shell: K2, L8, M18, N32, O18, P1
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f145d106s1
Thermal Properties of Gold
Phase: Solid
Melting point: 1337.33 K (1064 oC, 1947.52 oF)
Boiling point: 3243 K (2970 oC, 5378 oF)
Debye temperature: 170 K (-103.15 oC, -153.67 oF)
Fusion heat: 12.55 kJ/mol
Vaporization heat: 342 kJ/mol
Specific heat: 129 J/(kg K)
Molar heat capacity: 25.418 J/(mol.K)
Thermal expansion: 14.2 μm/(m∙K)
Thermal conductivity: 318 W/(m∙K)
Electrical properties of Gold
Electrical conductivity: 45×106 S/m
A Electrical resistivity: 22.14 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Gold
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -28×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000344
Mass magnetic susceptibility: -1,78×10-9 m3/kg
Molar magnetic susceptibility: -0.351×10-9 m3/mol
Physical Properties of Gold
Density: 19.30 g/cm3 (In solid) 17.31 g/cm3 (In Liquid at M.P)
Molar volume: 0.00001021 m3/mol
Tensile strength: 120 MPa
Young’s modulus: 79 GPa
Shear modulus: 27 GPa
Mohs Hardness: 2.5
Bulk modulus: 180 GPa
Poisson ratio: 0.4
Vicker hardness: 188-216 MPa
Brinell hardness: 188-245 MPa
Sound Speed: 2030 m/s
Atomic Properties of Gold
Oxidation states: -3, -2, -1, 1, 2, 3, 5
Valence Electrons: 5d10 6s1
Ion charge: Au3+ Au+
Ionization potential of an atom: 9.19
Ionization energies: 1st: 890 kJ.mol 2nd: 1980 kJ/mol
Ionic radius: 85 pm
Atomic radius: 144 pm (empirical)
Van der Waals: 166 Pm
Covalent radius: 136±6 pm
Filling Orbital: 5d10
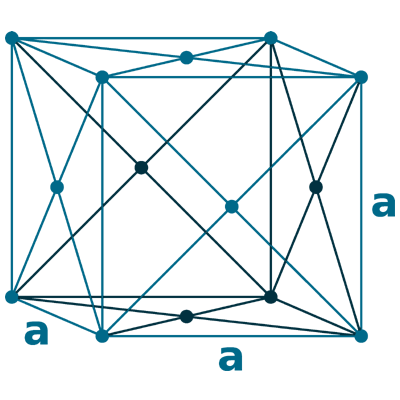
Crystal structure: Face centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 407.83, 407.83, 407.83 pm
Grid parameters: a=408 Å
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Gold
Electronegativity: 2.54 (pauling scale)
Valence: +5
Electron affinity: 222.8 kJ/mol
Nuclear Properties of Gold
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2S1/2
Neutron cross section (Brans): 98.7
Neutron Mass Absorption: 0.017
Isotopes: 195Au 196Au 197Au 198Au 199Au
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 195Au | Syn | – | 186.1 d |
| 196Au | Syn | – | 6.183 d |
| 197Au | 100 | 196.966 | Stable |
| 198Au | Syn | 197.967 | 2.695 d |
| 199Au | Syn | – | 3.169 d |
Chemical Reactions of Gold
The metal reacts chlorine & Bromine, and forming trihalides:
2 Au (s) + 3 Cl2 (g) → 2 AuCl3 (s) (gold (III) chloride)
2 Au (s) + 3 Br2 (g) → 2 AuBr3 (s) (gold (III) Bromide)
Reacts with iodine, It forms monohalide:
2 Au (s) + I2 (g) → 2 AuI (s) (gold (I) iodide)
Gold History
Naming: Au from Latin: aurum, meaning gold (from old English word geolo (yellow))
Discovery: (before 6000 BCE) In the Middle East
Gold Uses
Mostly Gold is used as bullion (used for trade on a market), however, it is also extensively used in Jewellery (in both Pure & alloy forms).
The purity of Gold is recognize by the term ‘Carat’, which indicates the amount of gold present in an alloy, where ‘24 carat’ is pure gold & it is very soft.
Mathematically,
1 carat is equals to 41.66 fineness,
24 carat gold equals to 999.99 fineness (Pure gold),
18 carat gold means 18 part of gold & 6 part of other metal (Gold alloy).
Gold alloys with 18 & 9 carat are commonly used , because they are more durable.
A Very thin sheets of gold (can be called as gold leaf) is used in art, for decoration, and as architectural ornament.
Gold is a good reflector of infrared, so it is used for coating certain space satellites, and on the windows of large building to reflect the heat of the Sun’s ray.
The metal is used in electroplating to cover another metal with a very thin layer of gold, which is used in cheap jewellery, gears of watches, artifitial limb joints, electrical connectors etc…
Because of its good conducting of electricity, it doesn’t corrode (break the contact), therefore, Gold electroplating is also used in electronic industry to protect their copper components & improve their solderability (used for joining), even the thin gold wires are used inside the computer chips to produce circuits.
In Medical field, the gold compounds is used to treat in some cases of arthritis, and The Dentists sometime use gold alloys for fillngs.
Gold nanoparticles are used as industrial catalyst, like Vinyl acetate (C4H6O2), which is used to make PVA (for glue, paint and resin).
Biological role of Gold: It is Non-toxic.
Abundance of Gold
Gold is one of the few elements to occur in a natural state (in veins & alluvial deposits), and is often separated from rocks and other minerals by mining & panning (wash gravel in a pan to separate out (gold)) operations.
Some minerals contains gold, such as: Calaverite (AuTe2), Krennerite (AuTe2, varing to 0.8 %to 0.2%), Nagyagite (Pb5Au(Te,Sb)4S5-8), Petzite (Ag3AuTe2), Sylvanite (Ag,Au)Te2, and the rare minerals are Maldonite (Au2Bi), Aurostibite (AuSb2), and also occurs in rare alloys Like Auricupride (Cu2Au), Novodneprite (AuPb3), Weishanite ((Au, Ag)3Hg2).
Seawater contains gold about or 0.1 to 2 mg/ton or 4 gm per million (1,000,000) tones of water.
Overall this is a huge amount of gold stored in the oceans, but because of the low concentration, attempts to reclaim this gold have always failed, where no method has been found yet for recovering gold from sea water profitably.
Gold is recovered from its ores by amalgamating, cyaniding, and smelting processes.
Refining is also frequently done by electrolysis (chemical decomposition).
Annual world wide production of Gold is around 2,800 tons, and World wide Reserve is around 187,200 tons.
6×10-8% (In Universe)
1.7×10-5% (In Meteorites)
1×10-7% (In Sun)
3.1×10-7% (In Earth’s Crust)
5×10-9% (In Oceans)
0.00001% (In Humans)








World’s Top 3 producers of Gold
1) China
2) Australia
3) Russia
World’s Top 3 Reserve holders of Gold
1) Australia
2) South Africa
3) Russia
Gold Price
Pure (99.995%) metal price is around $36,000-$42,000 per KG (KiloGram)
#Gold



I couldn’t refrain from commenting. Well written!