32 Ge (Germanium)

Pure germanium is a lustrous (retaining its luster in air at room temperature.), crystalline, brittle, and gray-white metalloid.
It is similar in chemical and physical properties to silicon.
It is a most important semiconductor.
AGermanium is stable in air and water, and is unaffected by alkalis and acids, except nitric acid.

Identity
CAS Number: CAS7440-56-4
CID Number: CID6326954
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSLY5200000
CONTENT INDEX
Basic Properties of Germanium
Pronunciation: Jar-may-nee-am
Appearance: Grayish-white
Mass Number: 73
Standard Atomic weight: 72.630 g/mol
Atomic number (Z): 32
Electrons: 32
Protons: 32
Neutrons: 41
Period: 4
Group: 14
Block: p
Element category: metalloids
Electrons per shell: K2, L8, M18, N4
Electron configuration: 1s22s22p63s23p63d104s24p2

Thermal Properties of Germanium
Phase: Solid
Melting point: 1211.40 K (938.25 oC, 1720.85 oF)
Boiling point: 3106 K (2833 oC, 5131 oF)
Debye temperature: 360 K (86.85 oC, 188.33 oF)
Fusion heat: 36.94 kJ/mol
Vaporization heat: 334 kJ/mol
Specific heat: 321.4 J/(kg K)
Molar heat capacity: 23.222 J/(mol.K)
Thermal expansion: 6 μm/(m∙K)
Thermal conductivity: 60.2 W/(m∙K)
Electrical properties of Germanium
Electrical conductivity: 2000 S/m
A Electrical resistivity: 1 Ω∙m
Band gap: 0.67 eV
A Electrical type: Semiconductor
Magnetic Properties of Germanium
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -76.84×10-6 cm3/mol
Volume magnetic susceptibility: -0.000000798
Mass magnetic susceptibility: -1.5×10-9 m3/kg
Molar magnetic susceptibility: -0.109×10-9 m3/mol
Physical Properties of Germanium
Density: 5.323 g/cm3 (In solid) 5.60 g/cm3 (In Liquid)
Molar volume: 0.000013645 m3/mol
Young’s modulus: 103 GPa
Shear modulus: 41 GPa
Mohs Hardness: 6.0
Bulk modulus: 75 GPa
Poisson ratio: 0.26
Vicker hardness: 8012.033 MPa
Brinell hardness: 7273.402 MPa
Sound Speed: 5400 m/s
Atomic Properties of Germanium
Oxidation states: 4, 3, 2, 1, 0, -1, -2, -3, -4
Valence Electrons: 4s2 4p2
Ion charge: Ge4+
The ionization potential of an atom: 7.85
Ionization energies: 1st: 762 kJ.mol 2nd: 1537.5 kJ/mol 3rd: 3302.1 kJ/mol
Ionic radius: 53 pm
Atomic radius: empirical: 122 pm
Van der Waals: 211 Pm
Covalent radius: 122 pm
Filling Orbital: 4p2
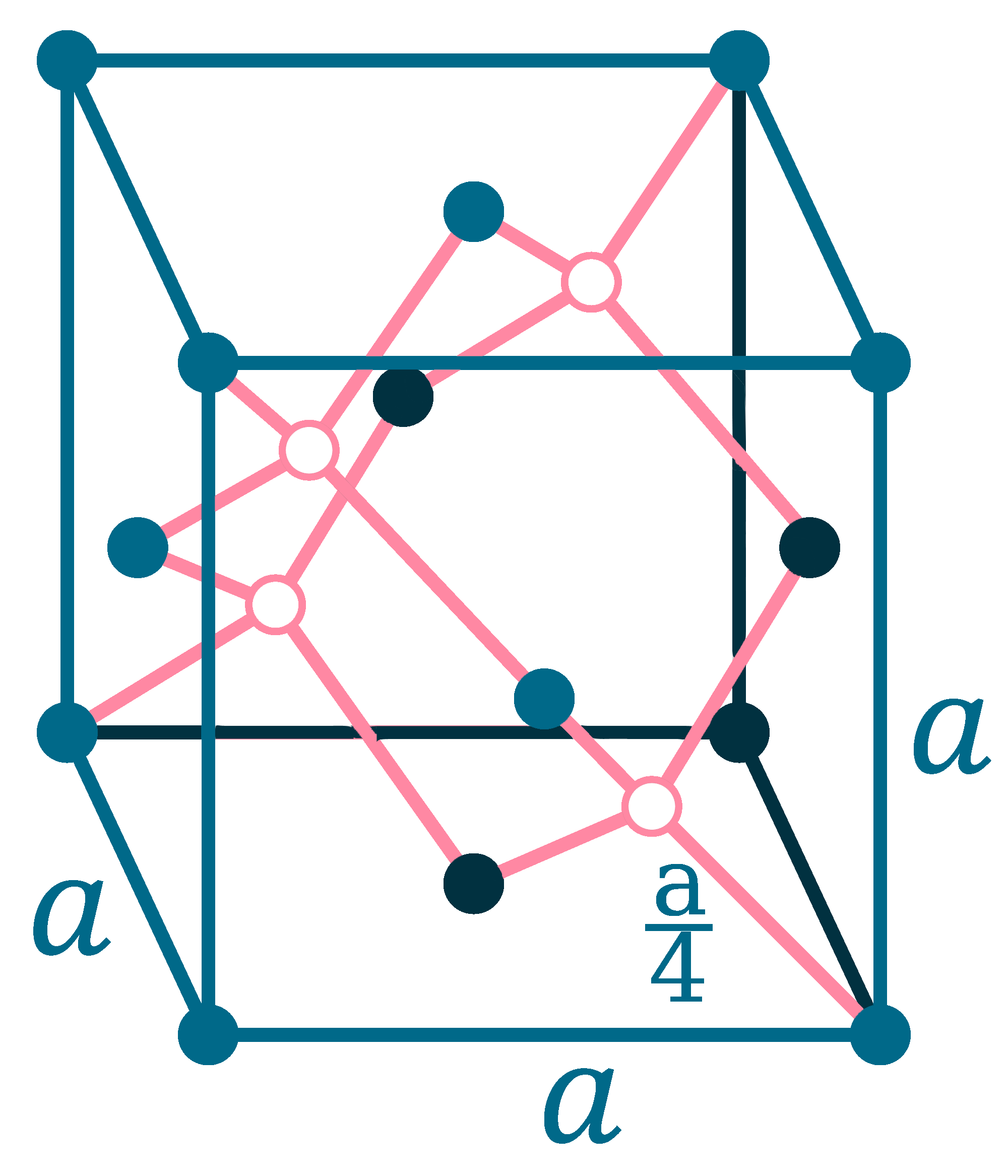
Crystal structure: Face centered diamond cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 565.75, 565.75, 565.75 pm
Grid parameters: a=5.660 Å
Space Group Name: Fm_3m
Space Group Number: 225
Reactivity of Germanium
Electronegativity: pauling scale: 2.01
Valence: +4
Electron affinity: 119 kJ/mol
Nuclear Properties of Germanium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 3P0
Neutron cross section (Brans): 2.2
Neutron Mass Absorption: 0.0011
Isotopes: 68Ge 70Ge 71Ge 72Ge 73Ge 74Ge 76Ge
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 68Ge | Syn | – | 270.8 d |
| 70Ge | 20.52 | 69.924 | Stable |
| 71Ge | Syn | – | 11.3 d |
| 72Ge | 27.45 | 71.922 | Stable |
| 73Ge | 7.76 | 72.923 | Stable |
| 74Ge | 36.52 | 73.921 | Stable |
| 76Ge | 7.75 | 75.921 | Stable |
Chemical Reactions of Germanium
At room temperature, it is stable in air, and At Red Heat , It reacts with O2, and forming a very thin protected layer of germanium dioxide on the surface of germanium.
Ge (s) + O2 (g) → GeO2 (s)
Germanium History
Naming: After Germany (homeland of the discoverer), Latin: Germania (Germany).
Prediction: Dmitri Mandeleev (1871)
Discovery: Clemens Winkler (1886)
Germanium Uses
Germanium is an important semiconductor, that is commonly doped with arsenic, gallium or other elements are used as a transistor element in thousands of electronic applications.
However, other semiconductors have replaced it in existing time.
Germanium and germanium oxide are transparent to the infrared, so they are used in infrared spectroscopes and other optical equipment, including extremely sensitive infrared detectors.
Germanium oxide has a high index of refraction and dispersion, This property is makes it suitable for use in wide-angle camera lenses and microscope objective lenses.
It is also finding many other applications including use as an alloying agent (adding 1% germanium in silver to stops it from tarnishing), as a phosphor in fluorescent lamps, and as a catalyst.
High purity germanium single crystal detectors can precisely identify radiation sources (e.g. for airport security)
Biological role of Germanium
AGermanium is Non-toxic element, but some germanium compounds have low toxicity in mammals, while being effective against some bacteria, which makes them useful as chemotherapeutic agents.
This has led some scientists to study their potential use in pharmaceuticals.
The estimated we intake daily is around 1 mg, and there have been claims that germanium could be beneficial to health, but this has never been scientifically proved.
A high intake of germanium is supposed to improve the immune system, bost the body’s oxygen supply, make a person feel more alive and destroy damaging free radicals.
But it has no nutritional or medical value and so it is consitute a risk to health, rather than a benefit.
AGermanium hydride and germanium tetrahydride (GeH4) are extremely flammable and even explosive when mixed with air.
The substance can be absorbed into the body by inhalation.
Abundance of Germanium
AGermanium are found in small quantities as the minerals germanite (contains 8%) and argyrodite (a sulfide of germanium and silver).
It’s ores are very rare.
It is also present in zinc ores, coal, and other minerals
Zine-refining techniques have led to production of crystalline germanium for semiconductor use with an impurity of only one part in 1010.
Commercial production of germanium is carried out by processing zinc smelter (process of converting zinc ores (contain zinc) into pure zinc) flue dust.
It can also be recovered from the by-products of combustion of certain coals.
Ultra-high pure germanium can be separated from other metals by fractional distillation of its volatile tetrachloride.
Annual world wide production is around 150 tons.
0.00002% (In Universe)
0.0021% (In Meteorites)
0.00002% (In Sun)
0.00014% (In Earth’s Crust)
6×10-9% (In Oceans)


World’s Top 3 producers of Germanium
1) China
2) Russia
3) Germany (likely)
#Germanium