31 Ga (Gallium)

Solid gallium is a blue-gray metal, and Highly pure metal has a beautiful silvery appearance.
It is soft enough to be cut with a knife.
It is one of four metals (mercury, cesium, and rubidium), which can be liquid near room temperature, has one of the longest liquid ranges of any metal.
AGallium is stable in air and water, but it reacts with and dissolves in acids and alkalis.
The metal expands 3.1 % on solidifying; So, it should not be stored in glass or metal containers, because they may break as the metal solidifies.

Identity
CAS Number: CAS7440-55-3
CID Number: CID5360835
DOT Hazard Class: 8
DOT Number: 2803
RTECS Number: RTECSLW8600000
CONTENT INDEX
Basic Properties of Gallium
Pronunciation: Gal-ee-am
Appearance: Silvery blue
Mass Number: 70
Standard Atomic weight: 69.723 g/mol
Atomic number (Z): 31
Electrons: 31
Protons: 31
Neutrons: 39
Period: 4
Group: 13
Block: p
Element category: Post-transition metal
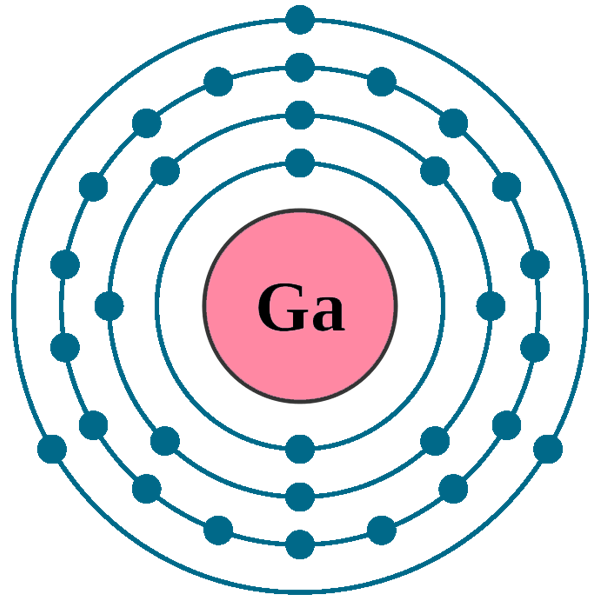
Electrons per shell: K2, L8, M18, N3
Electron configuration: 1s22s22p63s23p63d104s24p1
Thermal Properties of Gallium
Phase: Solid
Melting point: 302.91 K (29.76 oC, 85.57 oF)
Boiling point: 2673 K (2400 oC, 4352 oF)
Debye temperature: 240 K (-33.15 oC, -27.67 oF)
Fusion heat: 5.59 kJ/mol
Vaporization heat: 256 kJ/mol
Specific heat: 371 J/(kg K)
Molar heat capacity: 29.54 J/(mol.K)
Thermal expansion: 18 μm/(m∙K)
Thermal conductivity: 40.6 W/(m∙K)
Electrical properties of Gallium
Electrical conductivity: 7.1×106 S/m
A Electrical resistivity: 270 nΩ∙m
A Electrical type: Conductor
Critical point (Superconducting point): 1.083 K (-272.07 oC, -457.73 oF)
Magnetic Properties of Gallium
A Magnetic type: Diamagnetic
Magnetic susceptibility (xmol): -21.6×10-6 cm3/mol
Volume magnetic susceptibility: -0.0000177
Mass magnetic susceptibility: -3×10-9 m3/kg
Molar magnetic susceptibility: -0.209×10-9 m3/mol
Physical Properties of Gallium
Density: 5.91 g/cm3 (In solid) 6.095 g/cm3 (In Liquid)
Molar volume: 0.000011809 m3/mol
Young’s modulus: 9.8 GPa
Mohs Hardness: 1.5
Bulk modulus: GPa
Poisson ratio: 0.47
Brinell hardness: 56.8-68.7 MPa
Sound Speed: 2740 m/s
Atomic Properties of Gallium
Oxidation states: 3,2, 1, -1, -2, -4, -5
Valence Electrons: 4s2 4p1
Ion charge: Ga3+
The ionization potential of an atom: 5.97
Ionization energies: 1st: 578.8 kJ.mol 2nd: 1979.3 kJ/mol 3rd: 2963 kJ/mol
Ionic radius: 62 pm
Atomic radius: empirical: 135 pm
Van der Waals: 187 Pm
Covalent radius: 122±3 pm
Filling Orbital: 4p1
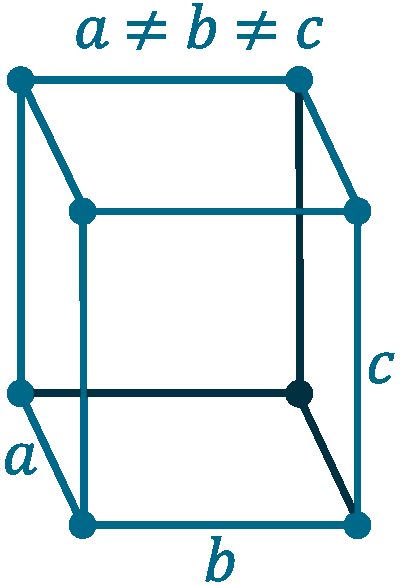
Crystal structure: Orthorhombic
Lattice angles: π/2, π/2, π/2
Lattice constant: 451.97, 765.8, 452.6 pm
Grid parameters: a=4.519 Å b= 7.658 Å c=4.526 Å
Space Group Name: Cmca
Space Group Number: 64
Reactivity of Gallium
Electronegativity: pauling scale: 1.81
Valence: +3
Electron affinity: 28.9 kJ/mol
Nuclear Properties of Gallium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2P1/2
Neutron cross section (Brans): 2.9
Neutron Mass Absorption: 0.0015
Isotopes: 66Ga 67Ga 68Ge 69Ge 70Ge 71Ge 72Ge 73Ge
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 66Ga | Syn | – | 9.5 h |
| 67Ge | Syn | – | 3.3 d |
| 68Ge | Syn | – | 1.2 h |
| 69Ge | 60.11 | 68.926 | Stable |
| 70Ga | Syn | – | 21 min |
| 71Ge | 39.89 | 70.925 | Stable |
| 72Ge | Syn | – | 14.1 h |
| 73Ge | Syn | – | 4.9 h |
Chemical Reactions of Gallium
AGallium reacts with sulfur forming gallium sulfide:
16 Ga (s) + 3 S8 (s) → 8 Ga2S3 (s)
Reaction of gallium tri-hydroxide (Ga(OH)3) with hydrogen sulfide (H 2S) at 747 °C, forming Gallium (III) sulfide:
2 Ga(OH)3 + 3 H2S → Ga2S3 + 6 H2O
React with ammonia at 1050 oC, and form Gallium nitride (GaN):
Li3Ga + N2 → Li3GaN2
Similarly It forms Gallium phosphide (GaP), Gallium arsenide (GaAs), and Gallium antimonide (GaSb).
These compounds have important semiconducting properties.
When heated to a high temperature, gallium(III) halides react with elemental gallium, and form gallium (I) halides:
2 Ga + GaCl3 ⇌ 3 GaCl (g)
Gallium History
Naming: After Gallia (Latin: France), Homeland of the discoverer
Prediction: Dmitri Mendeleev (1871)
Discovery: Lecoq de Boisbaudran (French chemist) (1875)
Gallium Uses
Because of high boiling point, It is used in some high temperature thermometers.
AGallium readily alloys with most metals, and has been used as a component in low-melting alloys.
The plutonium pits of nuclear weapons employ an alloy with agallium to stabilize the allotropes of plutonium.
Liquid gallium on porcelain and glass surfaces, forms a brilliant mirror when it painted or coated on glass.
Because of highly reflective, It is also called a liquid mirror.
But due to low melting point It melts near room temperature, so cannot used in mirror.
Gallium arsenide has a similar structure to silicon and is useful as a substitute of silicon for the electronics industry.
It is capable of converting electricity directly into coherent light and is used in red LEDs (light emitting diodes) and watches.
Solar panels on the Mars Exploration Rover contained gallium arsenide.
Gallium nitride is also a semiconductor, that properties make it very versatile.
It has mainly used in Blu-ray technology, mobile phones, blue and green LEDs, and pressure sensors for touch switches.

Biological role: It has Non-toxicity.
Abundance of Gallium
AGallium is often present in trace amounts in the minerals diaspore (AlO(OH)), germanite, sphalerite, bauxite and coal.
Some flue dusts from burning coal have been shown to contain 1.5% gallium.
It is mainly produced as a by-product of zinc refining.
The Metal is obtained by electrolysis of a solution of agallium(III) hydroxide in potassium hydroxide.
Annual world wide production is around 395 tons.
10×10-7% (In Universe)
76×10-5% (In Meteorites)
4×10-6% (In Sun)
0.0019% (In Earth’s Crust)
3×10-9% (In Oceans)





World’s Top 3 producers of Gallium
1) China
2) Germany
3) Kazakhstan
#Gallium



Do I have your permission do copy your website?
Bec making prints teens of each page is tiring me.
Printscreens not teens.
I’m sorry bad question.next pages…