27 Co (Cobalt Element)
Cobalt is a silver-white, hard, lustrous, brittle element, and resembling iron and nickel in appearance.
It can be magnetized ( Like Iron).
Cobalt tends to exist as a mixture of two allotropes over a wide temperature range.
Its physical properties is similar to iron and nickel.
The element is active chemically, and forming many compounds.
Cobalt is stable in air and unaffected by water, but is slowly attacked by dilute acids.

Identity
CAS Number: CAS7440-48-4
CID Number: CID104730
DOT Hazard Class: 4.1
DOT Number: 3089
RTECS Number: RTECSGF8750000
CONTENT INDEX
Basic Properties of Cobalt Element
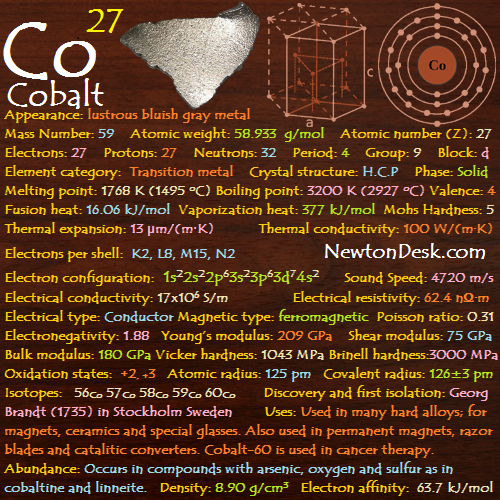
Appearance: Hard lustrous bluish gray metal
Mass Number: 59
Standard Atomic weight: 58.933 g/mol
Atomic number (Z): 27
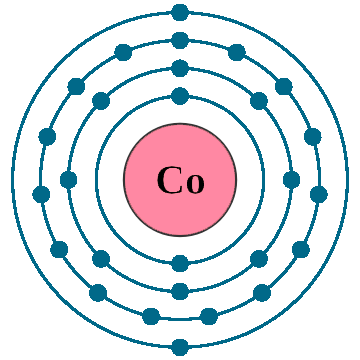
Electrons: 27
Protons: 27
Neutrons: 32
Period: 4
Group: 9
Block: d
Element category: Transition metal
Electrons per shell: K2, L8, M15, N2
Electron configuration: 1s22s22p63s23p63d74s2
Thermal Properties of Cobalt Element
Phase: Solid
Melting point: 1768 K (1495 oC, 2723 oF)
Boiling point: 3200 K (2927 oC, 5301 oF)
Debye temperature: 385 K (111.85 oC, 233.33 oF)
Fusion heat: 16.06 kJ/mol
Vaporization heat: 377 kJ/mol
Specific heat: 421 J/(kg K)
Molar heat capacity: 24.81 J/(mol.K)
Thermal expansion: 13 μm/(m∙K)
Thermal conductivity: 100 W/(m∙K)
Electrical properties of Cobalt
Electrical conductivity: 17×106 S/m
A Electrical resistivity: 62.4 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Cobalt Element
A Magnetic type: ferromagnetic
Curie point: 1394 K (1120.85 oC, 2049.53 oF) (above which Ferromagnetism vanishes)
Magnetic susceptibility (xmol): +1860×10-6 cm3/mol
Physical Properties of Cobalt
Density: 8.90 g/cm3 (In solid) 8.86 g/cm3 (In Liquid at M.P)
Molar volume: 0.00000662 m3/mol
Young’s modulus: 209 GPa
Shear modulus: 75 GPa
Mohs Hardness: 5.0
Bulk modulus: 180 GPa
Poisson ratio: 0.31
Vicker hardness: 1043 MPa
Brinell hardness: 470-3000 MPa
Sound Speed: 4720 m/s
Atomic Properties of Cobalt Element
Oxidation states: -3, -1, +1, +2, +3, +4, +5
Valence Electrons: 3d7 4s2
Ion charge: Co2+ Co3+
The ionization potential of an atom: 7.81
Ionization energies: 1st: 760.4 kJ.mol 2nd: 1648 kJ/mol 3rd: 3232 kJ/mol
Ionic radius: 74.5 pm
Atomic radius: empirical: 125 pm
Van der Waals: 192 Pm
Covalent radius: 126±3 pm (Low spin), 150±7 pm (High spin)
Filling Orbital: 3d7
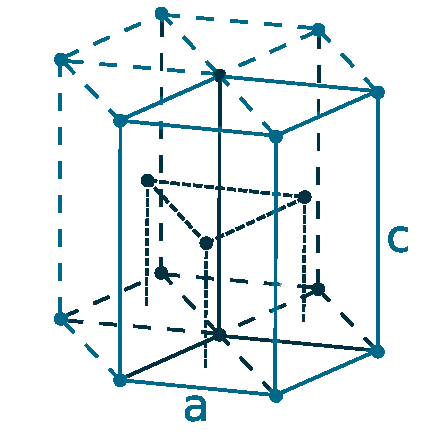
Crystal structure: Hexagonal close packed
Lattice angles: π/2, π/2, 2π/3
Lattice constant: 250.5, 250.5, 408.9 pm
Grid parameters: a=2.505 Å c=4.089 Å
Attitude c/a: 1.632
Space Group Name: P63/mmc
Space Group Number: 194
Reactivity of Cobalt Element
Electronegativity: pauling scale: 1.88
Valence: +4
Electron affinity: 63.7 kJ/mol
Nuclear Properties of Cobalt
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 4F9/2
Neutron cross section (Brans): 37.2
Neutron Mass Absorption: 0.021
Isotopes: 56Co 57Co 58Co 59Co 60Co
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 56Co | Syn | – | 77.27 d |
| 57Co | Syn | – | 271.79 d |
| 58Co | Syn | – | 70.86 d |
| 59Co | 100 | 58.933 | Stable |
| 60Co | Syn | – | 5.2714 y |
Chemical Reactions of Cobalt Element
It does not react readily with air. However, on heating the oxide Co3O4 is formed:
3Co (s) + 4 O2 (g) → 2 Co3O4 (s)
If the reaction is carried out above 900°C, the cobalt(II) oxide CoO is formed:
2 Co (s) + O2 (g) → 2 CoO (s)
Cobalt does not react with Nitrogen.
Water has little effect upon cobalt metal, The reaction between red hot cobalt metal and steam produces cobalt(II) oxide, CoO.
2 Co (s) + O2 (g) → 2 CoO (s)
The metal reacts with bromine, and forming:
Co (s) + Br2 (I) → CoBr2 (s) [green] (Cobalt (II) bromide)
The metal also reacts with chloride and iodide, but as a synthetic pathway other reactions are used:
Co (s) + Cl2 (g) → CoCl2 (s) [blue] (Cobalt (II) chloride)
Co (s) + I2 (s) → CoL2 (s) [blue-black] (Cobalt (II) Iodide)
Co(II) forms complexes with Cl–:
[Co(H2O)6]2+ (aq) + 4 Cl– (aq) → [CoCl4]2- (aq) + 6 H2O (I)
Cobalt metal dissolves slowly in dilute sulphuric acid, and forming aquated solutions containing the Co(II) ion together with hydrogen gas, he actual occurrence of Co(II) in aqueous solution is as the complex ion [Co(OH2)6]2+.
Co (s) + H2SO4 (aq) → Co2+ (aq) + SO42- (aq) + H2 (g)
Co(II) reacts with HCl(aq) under strongly acidic conditions, and forming tetrahedral chloro complexes:
[Co(H2O)6]2+ (aq) + 4 Cl– (aq) → [CoCl4]2- (aq) [deep blue] + 6 H2O (I)
Cobalt History
Naming: German: kobalt or kobold (evil spirit); Greek: cobalos (mines).
Discovery and first isolation: Georg Brandt (1735) in Stockholm Sweden
Cobalt Uses
Cobalt element can be magnetized (like iron), so it is used to make magnets, It is alloyed with iron, nickel and other metals to make Alnico (Al, Ni, Co), an alloy of unusual magnetic strength with many important uses.
Stellite alloys (containing cobalt, chromium, and tungsten) are used for high-speed, heavy-duty, high temperature cutting tools, and dies.
Other alloys of cobalt are used in magnetic steels, stainless steels, and in jet turbine and gas turbine generators (where high-temperature strength is important).
The metal is used in electroplating because of its attractive appearance, hardness and resistance to corrosion or oxidation.
Cobalt salts have been used for centuries to produce brilliant and permanent blue colours in paint, porcelain (a white vitrified translucent ceramic), glass, tiles, pottery and enamels.
Its rich blue colour is also known as Sèvres blue and Thénard blue.
A aqueous solution of the cobalt chloride (CoCl2) with added glycerol is almost colourless (very pale pink) , and it was used to write secret massages, It is also popular as invisible ink.
It would read by simply heated the paper and the hidden writing appeared, Because the heating drove off the water and glycerol molecules that surround the cobalt, and chloride ions moved in to take their place, giving dark blue CoCl42- ions.
Cobalt carefully used in the form of the chloride (CoCl2), sulfate (CoSO4), acetate (Co(CH3COO)2) , or nitrate (Co(NO3)2) has been found effective in correcting a certain mineral deficiency disease in animals.
Biological role of Cobalt Element
Cobalt element is essential to the metabolism of all animals, and It forms part of the active site of vitamin B12.
The amount we need is very small, and an uptake of 0.20 mg/kg a day (body contains only about 1 milligram) is recommended because they have no other source of vitamin B12.
In large doses of cobalt is carcinogenic.
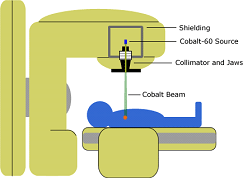
Cobalt-60 is a radioactive isotope, and It is an important source of gamma-rays.
It is widely used in cancer treatment, to irradiate food to preserve it, and as a tracer and for radiotherapy.
also used in Dirty bomb or Cobalt Bomb kind of Salted bomb “Claimed by Russia to Ukraine could use in Ukraine Russia War”
Abundance of Cobalt
Cobalt is found in the minerals Cobaltite (CoAsS), Skutterudite (CoAs3)and Erythrite (Co3(AsO4)2·8H2O), and is often associated with nickel, silver, lead, copper, and iron ores, from which it is formed as a by-product of refining.
It is also present in meteorites.
The U.S. Geological Survey has announced that the bottom of the north central Pacific Ocean may have a huge reserve of several transition metals (including many tonnes of cobalt) deposits on the floors of the deepest in water close to the the Hawaiian Islands and other U.S. Pacific territories.
Annual world wide production is around 2,00,000 metric tons, and World wide Reserve is around 7,100,000 metric tons.
0.0003% (In Universe)
0.059% (In Meteorites)
0.0004% (In Sun)
0.003% (In Earth’s Crust)
8×10-9% (In Oceans)
2×10-6% (In Humans)



World’s Top 3 producers of Cobalt
1) DRC
2) China
3) Zambia
World’s Top 3 Reserve holders of Cobalt
1) DRC
2) Australia
3) Cuba
#Cobalt