55 Cs (Cesium)

Cerium is a silvery-gold, soft and ductile.
It is the most electropositive and alkaline element.
Cerium, mercury, and gallium are the only metals that are liquid at room temperature.
Cerium metal rapidly oxidized in air and can form dangerous superoxide on its surface.
It reacts Explosively with cold water, and reacts with ice at temperatures above -116 oC.

Identity
Alternative name: Caesium (British spelling and IUPAC spelling)
CAS Number: CAS7440-46-2
CID Number: CID5354618
EINECS Number: 231-155-4
RTECS Number: RTECSFK9225000
DOT Hazard Class: 4.3
DOT Number: 1407
CONTENT INDEX
Basic Properties of Cesium
Pronunciation: See-zee-am
Appearance: pale gold
Mass Number: 133
Standard Atomic weight: 132.905 g/mol
Atomic number (Z): 55
Electrons: 55
Protons: 55
Neutrons: 78
Group: 1
Period: 6
Block: s
Element category: Alkali metal
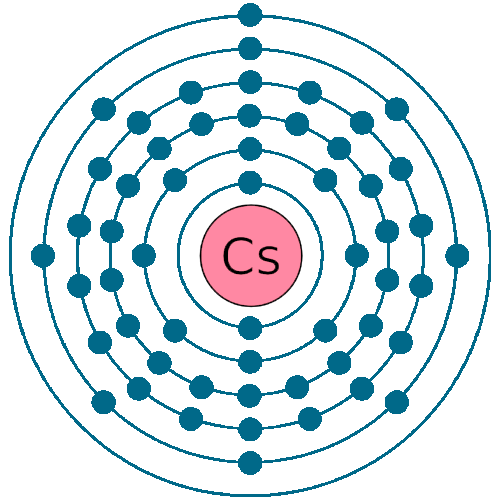
Electrons per shell: K2, L8, M18, N18, O8, P1
Electron configuration: 1s22s22p63s23p63d104s24p64d105s25p64f56s1
Thermal Properties of Cesium
Phase: Solid
Melting point: 301.7 K (28.5 oC, 83.3 oF)
Boiling point: 944 K (671 oC, 1240 oF)
Debye temperature: 39.2 K (-233.95 oC, -389.11 oF)
Critical temperature: 1938 K (1664.85 oC, 3028.73 oC)
Critical Pressure: 9.4 MPa (92.77 Atm)
Fusion heat: 2.09 kJ/mol
Vaporization heat: 63.9 kJ/mol
Specific heat: 242 J/(kg K)
Molar heat capacity: 32.210 J/(mol.K)
Thermal expansion: 97 μm/(m∙K)
Thermal conductivity: 35.9 W/(m∙K)
Electrical properties of Cesium
Electrical conductivity: 5×106 S/m
A Electrical resistivity: 205 nΩ∙m
A Electrical type: Conductor
Magnetic Properties of Cesium
Magnetic type: Paramagnetic
Volume magnetic susceptibility: -0.00000526
Mass magnetic susceptibility: -2.8×10-9 m3/kg
Molar magnetic susceptibility: -0.372×10-9 m3/mol
Physical Properties of Cesium
Density: 1.93 g/cm3 (In solid) 1.843 g/cm3 (In Liquid)
Molar volume: 0.00007073 m3/mol
Young’s modulus: 1.7 GPa
Mohs Hardness: 0.2
Bulk modulus: 1.6 GPa
Brinell hardness: 0.14 MPa
Atomic Properties of Cesium
Oxidation states: +1, -1
Valence Electrons: 6s1
Ion charge: Cs+
Ionization potential of an atom: 3.88
Ionization energies: 1st: 375.7 kJ.mol 2nd: 2234.3 kJ/mol 3rd: 3400 kJ/mol
Ionic radius: 167 pm
Atomic radius: 343 pm (Van der Waals)
Covalent radius: 244±11 pm
Filling Orbital: 6s1
Crystal structure: Body-centered cubic
Lattice angles: π/2, π/2, π/2
Lattice constant: 614.1, 614.1, 614.1 pm
Grid parameters: 6.140 Å
Space Group Name: lm_3m
Space Group Number: 229

Reactivity of Cesium
Electronegativity: pauling scale: 0.79
Valence: +1
Electron affinity: 45.5 kJ/mol
Nuclear Properties of Cesium
Half Life: Stable (Infinity)
Lifetime: Stable (Infinity)
Quantum Number: 2S1/2
Neutron cross section (Brans): 29
Neutron Mass Absorption: 0.0077
Isotopes: 133Cs 134Cs 135Cs 137Cs
| Isotope | Abundance (%) | Atomic Mass g/mol | Half Life (t1/2) |
| 133Cs | 100 | 132.905 | Stable |
| 134Cs | Syn | – | 2.0648 y |
| 135Cs | Trace | – | 2.3×106 y |
| 137Cs | Syn | – | 30.17 y |
Chemical Reactions
The metal is highly reactive and Pyrophoric (ignite spontaneously in air or below 55 oC), Formation of cesium superoxide.
Cs (s) + O2 (g) → CsO2 (s) [orange]
Reacts explosively with water even at low temperatures (form Cesium hydroxide and hydrogen gas). However, cesium-water explosion is less powerful than sodium-water explosion:
2 Cs (s) + 2 H2O (l) → 2 CsOH (aq) + H2 (g)
Reacts with hydrogen (forming a cesium hydride):
2 Cs (s) + H2 (g) → 2 CsH (s)
The metal reacts vigorously with all Halogens and form Cesium halides:
2 Cs (s) + 3 F2 (g) → 2 CsF3 (s) (Cesium fluoride)
2 Cs (s) + 3 Cl2 (g) → 2 CsCl3 (s) (Cesium chloride)
2 Cs (s) + 3 Br2 (g) → 2 CsBr3 (s) (Cesium bromide)
2 Cs (s) + 3 I2 (g) → 2 CsI3 (s) (Cesium iodide)
Dissolves readily in dilute sulfuric acid to form Solutions containing Cs (l) ions (aquated):
2 Cs (s) + H2SO4 (aq) → 2 Cs+ (aq) + SO42– (aq) + H2 (g)↑
Cesium History
Naming: From Latin caesius (sky blue), Its compounds burn with blue or violet colour.
Discovery: Robert Bunsen and Gustav Kirchhoff (1860) Heidelberg, Germany
First isolation: Carl Setterberg (1882)
Cesium Uses
Cesium is used in industry as a catalyst promoter, in vacuum tubes and light bulbs to remove traces of oxygen, and in radiation monitoring equipment.
Caesium nitrate is used to make special optical glasses.
Caesium chloride is used in optical instruments, photoelectric cells, and in increasing the sensitivity of electron tubes.
A Caesium vapour is used in many common magnetometers
A Cesium is mainly used in (Cesium clock) atomic clock. These clocks are a vital part of the internet and mobile phone networks, as well as Global Positioning System (GPS) satellites. The most accurate realization of a unit that mankind has yet achieved. Some cesium clocks are accurate to 1 second in 15 million years. The electron echo frequency of the cesium atom is “1 second = 9,192, 631,770 cycles of the standard Cs-133 transition”
A Caesium is more recently used in ion propulsion systems.
Biological role: Cesium compounds, such as cesium chloride, are low hazard.
Abundance of Cesium
Caesium is found in the minerals pollucite and lepidolite.
They are silicate magmas cooled from granites.
Annual world wide production is around 20 tons, mainly from the Bernic lake (Canada) and a little from Zimbabwe and South-west Africa
8×10-8% (In Universe)
1.4×10-5% (In Meteorites)
8×10-7% (In Sun)
0.00019% (In Earth’s Crust)
5×10-8% (In Oceans)
2×10-6%(In Humans)


#cesium


